CD44 variant exons induce chemoresistance by modulating cell death pathways
- PMID: 40114966
- PMCID: PMC11924683
- DOI: 10.3389/fcell.2025.1508577
CD44 variant exons induce chemoresistance by modulating cell death pathways
Abstract
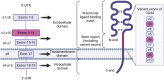
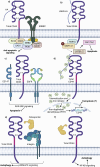
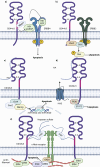
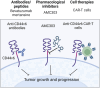
Cancer chemoresistance presents a challenge in oncology, often leading to treatment failure and disease progression. CD44, a multifunctional cell surface glycoprotein, has garnered attention for its involvement in various aspects of cancer biology. Through alternative splicing, CD44 can form isoforms with the inclusion of only standard exons, typical for normal tissue, or with the addition of variant exons, frequently expressed in cancer tissue and associated with chemoresistance. The functions of CD44 involved in regulation of cancer signaling pathways are being actively studied, and the significance of specific variant exons in modulating cell death pathways, central to the response of cancer cells to chemotherapy, begins to become apparent. This review provides a comprehensive analysis of the association of CD44 variant exons/total CD44 with clinical outcomes of patients undergoing chemotherapy. The role of CD44 variant exons v6, v9 and others with a significant effect on patient chemotherapy outcomes by means of key cellular death pathways such as apoptosis, ferroptosis and autophagy modulation is further identified, and their impact on drug resistance is highlighted. An overview of clinical trials aimed at targeting variant exon-containing isoforms is provided, and possible directions for further development of CD44-targeted therapeutic strategies are discussed.
Keywords: CD44 variant exon 6; CD44 variant exon 9; apoptosis; autophagy; cancer; chemoresistance; ferroptosis.
Copyright © 2025 Yanova, Stepanova, Maltseva and Tonevitsky.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures




Similar articles
-
Restricted patterns of CD44 variant exon expression in human papillary thyroid carcinoma.Cancer Res. 1996 Mar 1;56(5):1037-42. Cancer Res. 1996. PMID: 8640758
-
Distribution of CD44 variant isoforms in human skin: differential expression in components of benign and malignant epithelia.J Cutan Pathol. 1995 Dec;22(6):536-45. doi: 10.1111/j.1600-0560.1995.tb01148.x. J Cutan Pathol. 1995. PMID: 8835172
-
Regulated expression of exon v6 containing isoforms of CD44 in man: downregulation during malignant transformation of tumors of squamocellular origin.J Cell Biol. 1993 Jul;122(2):431-42. doi: 10.1083/jcb.122.2.431. J Cell Biol. 1993. PMID: 8320265 Free PMC article.
-
CD44: structure, function, and association with the malignant process.Adv Cancer Res. 1997;71:241-319. doi: 10.1016/s0065-230x(08)60101-3. Adv Cancer Res. 1997. PMID: 9111868 Review.
-
CD44 and its implication in neoplastic diseases.MedComm (2020). 2024 May 23;5(6):e554. doi: 10.1002/mco2.554. eCollection 2024 Jun. MedComm (2020). 2024. PMID: 38783892 Free PMC article. Review.
Cited by
-
Combined Hyaluronic Acid Nanobioconjugates Impair CD44-Signaling for Effective Treatment Against Obesity: A Review of Comparison with Other Actors.Int J Nanomedicine. 2025 Aug 21;20:10101-10126. doi: 10.2147/IJN.S529250. eCollection 2025. Int J Nanomedicine. 2025. PMID: 40859950 Free PMC article. Review.
-
The Capability to Undergo ACSL4-Mediated Ferroptosis Is Acquired During Brown-like Adipogenesis and Affected by Hypoxia.Cells. 2025 Aug 13;14(16):1247. doi: 10.3390/cells14161247. Cells. 2025. PMID: 40862726 Free PMC article.
References
-
- Anand V., Khandelwal M., Appunni S., Gupta N., Seth A., Singh P., et al. (2019). CD44 splice variant (CD44v3) promotes progression of urothelial carcinoma of bladder through Akt/ERK/STAT3 pathways: novel therapeutic approach. J. Cancer Res. Clin. Oncol. 145 (11), 2649–2661. 10.1007/s00432-019-03024-9 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

