Treatment of non-Hodgkin lymphoma with point-of-care manufactured CAR T cells: a dual institution, phase 1 trial
- PMID: 40115173
- PMCID: PMC11925540
- DOI: 10.1016/j.eclinm.2025.103138
Treatment of non-Hodgkin lymphoma with point-of-care manufactured CAR T cells: a dual institution, phase 1 trial
Abstract
Background: Point-of-care manufacture of chimeric antigen receptor (CAR)-T cells can significantly reduce the time from apheresis to infusion. We conducted a dual-institution phase I trial aimed evaluating the safety and feasibility of this manufacturing model.
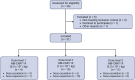
Methods: CASE 2417 was a phase I clinical trial. Adults with relapsed or refractory CD19 positive non-Hodgkin lymphoma (R/R NHL) treated with ≥2 prior systemic therapies were eligible. MB-CART-19 is an anti-CD19 CAR T-cell product manufactured using the CliniMACS Prodigy device with 4-1BB and CD3ζ costimulatory domains. Lymphodepletion included fludarabine 25 mg/m2 for 3 days and cyclophosphamide 60 mg/kg for 1 day. Prophylactic tocilizumab was allowed. Three dose levels (0.5, 1.0 and 2.0 × 106 cells/kg) were tested using a 3 + 3 dose-escalation schema. The primary outcome of this study was to determine the safety as defined by the dose limiting toxicities of MB-CART-19 in patients with relapsed and refractory NHL, co-primary outcome was determining the phase 2 dose of MB-CART-19. Secondary outcomes include defining the toxicity profile and to evaluate the initial efficacy of MB-CART-19 against relapsed or refractory NHL. This study was registered in ClinicalTrials.govNCT03434769.
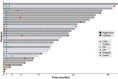
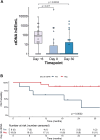
Findings: Thirty-one patients were enrolled between July 2018 and January 2021. Twenty-four (77%) had aggressive lymphoma, 7 (24%) had indolent lymphoma (follicular lymphoma and marginal zone lymphoma). The median number of previous therapies was 5 (range 2-13, interquartile range [IQR] 3-5). All enrolled patients received MB-CART-19. Median apheresis to infusion time was 13 days (range 9-20, IQR 9-13). One dose limiting toxicity (DLT) was observed in dose escalation (fatal cytokine release syndrome [CRS]), whereas one patient died in dose expansion secondary to hemophagocytic syndrome. Both deaths were considered treatment-related. Twenty (65%) patients had CRS, three (10%) grade ≥3. Ten patients (32%) experienced immune effector cell neurotoxicity syndrome (ICANS), four (13%) grade ≥3. Neutropenia (n = 28, 90%), thrombocytopenia (n = 15, 48%) and anaemia (n = 13, 42%) were the most frequent grade ≥3 adverse events. Twenty-five out of 29 (86%, 95% confidence interval [CI]: 68-96%) response-evaluable patients had disease response and 22 (76%, 95% CI: 56-90%) had complete response; the overall and complete response rates for response-evaluable aggressive lymphoma patients (n = 22) were 82% (n = 18, 95% CI: 60-95%) and 73% (n = 16, 95% CI: 50-89%). Median follow up was 24.5 (IQR 17-32) months, median progression free survival (PFS) was 26 months (95% CI: 19-not reached [NR]) and median PFS was not reached (95% CI: 25 months-NR). Two-year estimates of PFS and overall survival (OS) were 63% (95% CI: 47-83%) and 68% (95% CI: 52-88%), respectively. Median PFS was 26 months (95% CI: 7-NR) for aggressive lymphoma patients with 2-year PFS estimate of 53% (95% CI: 36-78%), while median OS had not been reached for aggressive lymphoma patients (95% CI: 19 months-NR), and 2-year OS estimate was 60% (95% CI: 43-85%).
Interpretation: Point-of-care CAR T-cell manufacture was feasible and replicable across sites. MB-CART-19 has a safety profile comparable to other CAR T-cell products and high response rates. The recommended phase 2 dose is 2 × 106 MB-CART-19 cells/kg. Short CAR T-cell manufacturing time permits treatment of patients with rapidly progressive lymphoma, a group of patients with high risk disease for whom access to autologous immune effector cellular therapies is usually limited.
Funding: This clinical trial was funded through University Hospitals Seidman Cancer Center and Washington University School of Medicine Institutional Funds. Correlative analyses were funded in part by the European Union-Next Generation EU-NRRP M6C2-Investment 2.1 Enhancement and strengthening of biomedical research in the NHS (project #PNRR-MAD-2022-12376059), and the Italian Ministry of Health Ricerca Finalizzata 2019 (project #RF-2019-12370243).
Keywords: Chimeric antigen receptor T cell; Immune effector cell therapy; Lymphoma; Phase I; Point-of-care manufacture.
© 2025 The Author(s).
Conflict of interest statement
AG has received honoraria from Kite, a Gilead Company; has provided consultancy for Amgen, Atara, Bristol Myers Squibb, CRISPR therapeutics, Kite, Cargo Therapeutics, and Wugen Inc.; and has received research funding from Amgen, Genentech, Secura Bio, and Kite. PFC has received research funding from Abbvie, ADC Therapeutics, Genentech, Genmab and Recordati Rare diseases. Has provided consultancy to Recordati Rare Diseases, Arvinas. Has served on advisory boards for ADC Therapeutics, Abbvie, Autolus, BMS, Genmab, Genentech, Kite, Luminary Thereapeutics, Sobi, Synthekine, Novartis, Takeda. ZJ is a current employee of Poseida Therapeutics, Inc. DS, WK, MK, BD are current or former employees of Lentigen Technology, Inc. a Miltenyi Biotec Company, Gaithersburg, Maryland. DS has patents planned, issued or pending related to this work. EZ has served in data safety monitoring board for Biodesix, Inc. Honorarium from American Urological Association for participation on MCURe Workshop. LM has served on the speaker's bureau of Incyte Pharmaceuticals and served on advisory boards for Pfizer. EG has served on the speaker's bureau of Eli Lilly, has participated in OncLive educational events and served on an advisory board for Incyte. RO has served on scientific advisory board of Umoja Biopharma and Galapagos NV.
Figures




References
-
- Mian A., Hill B.T. Brexucabtagene autoleucel for the treatment of relapsed/refractory mantle cell lymphoma. Expert Opin Biol Ther. 2021;21:1–7. - PubMed
-
- Jacobson C.A., Chavez J.C., Sehgal A.R., et al. Axicabtagene ciloleucel in relapsed or refractory indolent non-Hodgkin lymphoma (ZUMA-5): a single-arm, multicentre, phase 2 trial. Lancet Oncol. 2022;23:91–103. - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

