MiR-326: Role and significance in brain cancers
- PMID: 40115178
- PMCID: PMC11925037
- DOI: 10.1016/j.ncrna.2025.02.006
MiR-326: Role and significance in brain cancers
Abstract
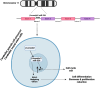
MicroRNAs (miRNAs) are small non-coding RNAs that act as critical regulators of gene expression by repressing mRNA translation. The role of miRNAs in cell physiology spans from cell cycle control to cell proliferation and differentiation, both during development and in adult tissues. Accordingly, dysregulated expression of miRNAs has been reported in several diseases, including cancer, where miRNAs can act as oncogenes or oncosuppressors. Of note, miRNA signatures are also under investigation for classification, diagnosis, and prognosis of cancer patients. Brain tumours are primarily associated with poor prognosis and high mortality, highlighting an urgent need for novel diagnostic, prognostic, and therapeutic tools. Among miRNAs investigated in brain tumours, miR-326 has been shown to act as a tumour suppressor in adult and paediatric brain cancers. In this review, we describe the role of miR-326 in malignant as well as benign cancers originating from brain tissue. In addition, since miR-326 expression can be regulated by other non-coding RNA species, adding a further layer of regulation in the cancer-promoting axis, we discuss this miRNA's role in targeted therapy for brain cancers.
Keywords: ARRB1; Circular RNAs (circRNAs); Gliomas; Long non-coding RNAs (lncRNAs); Medulloblastoma; MiR-326; Pituitary adenomas.
© 2025 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.AbbreviationsmiRNAsMicroRNAsARRB1β-arrestinsATPadenosine triphosphateceRNAcompetitive endogenous RNAcirc_0029696kinetochore associated complex subunit 3circ_0082374Circ-RNA carboxypeptidasecirc-RNAscircular RNAscirc-SKA3circ-RNA spindleCNSCentral nervous systemCSCsCancer stem cellsDDL1delta-like ligandE2F1E2F transcription factor 1FGF1Fibroblast growth factor 1GBMGlioblastomaGCPsCerebellar granule cell precursorsGHGrowth hormoneGPCRsG protein coupled receptorsGSCsGlioma-stem cellsHGGsAdult high-grade gliomasHHHedgehogHMGA2High mobility group AT-hook 2HPGDSHematopoietic prostaglandin D synthaseID3Inhibitor of differentiation 3IDHIsocytrate deidrogenaseJAGJagged ligandLGGLow-grade gliomaslnc-RNAsLong non-coding RNAsMAPKMitogen-activated protein kinaseMBMedulloblastomaMREsMiRNA response elementsNOB1Nin One Binding ProteinNSCsCerebellar neural stem cellsPAPituitary adenomasPI3KPhosphoinositide 3-KinasePKM2Pyruvate Kinase 2PTCH1Transmembrane receptor patched 1PTENPhosphatase and tensin homologSGMS1Sphingomyelin synthase 1SHHSonic hedgehogSIRT1Sirtuin 1SMAD3Mothers against decapentaplegic family 3SMOTransmembrane protein smoothenedSNHG9Small nucleolar RNA host gene 9SOX9Sex-determining region Y (SRY)-box 9 proteinTFTranscription factorTMZTemozolomideVDRVitamin D receptorWHOWorld Health OrganizationWHOWorld Health OrganizationWnt7BWnt family member 7BZIC3Zinc-finger in cerebellum 3
Figures
Similar articles
-
The Role of microRNAs, Long Non-coding RNAs, and Circular RNAs in Cervical Cancer.Front Oncol. 2020 Feb 20;10:150. doi: 10.3389/fonc.2020.00150. eCollection 2020. Front Oncol. 2020. PMID: 32154165 Free PMC article. Review.
-
Dysregulated expression and functions of microRNA-330 in cancers: A potential therapeutic target.Biomed Pharmacother. 2022 Feb;146:112600. doi: 10.1016/j.biopha.2021.112600. Epub 2021 Dec 27. Biomed Pharmacother. 2022. PMID: 34968919
-
Interplay between lncRNA/miRNA and Wnt/ß-catenin signaling in brain cancer tumorigenesis.EXCLI J. 2023 Nov 28;22:1211-1222. doi: 10.17179/excli2023-6490. eCollection 2023. EXCLI J. 2023. PMID: 38204968 Free PMC article. Review.
-
Diagnostic, grading and prognostic role of a restricted miRNAs signature in primary and metastatic brain tumours. Discussion on their therapeutic perspectives.Mol Genet Genomics. 2022 Mar;297(2):357-371. doi: 10.1007/s00438-021-01851-5. Epub 2022 Jan 21. Mol Genet Genomics. 2022. PMID: 35064290
-
Methylation of the miR-126 gene associated with glioma progression.Fam Cancer. 2016 Apr;15(2):317-24. doi: 10.1007/s10689-015-9846-4. Fam Cancer. 2016. PMID: 26463235
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

