An Expanded Access Protocol of RNS60 in Amyotrophic Lateral Sclerosis
- PMID: 40116017
- PMCID: PMC12138494
- DOI: 10.1002/mus.28398
An Expanded Access Protocol of RNS60 in Amyotrophic Lateral Sclerosis
Abstract
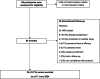
Aims: RNS60 is an investigational product in clinical development for amyotrophic lateral sclerosis (ALS). RNS60 slowed disease progression in the ALS SOD1G93A mouse model and was safe and well tolerated both in an open-label pilot study and a randomized, placebo-controlled, multicenter phase 2 trial in people living with ALS. The objective of this ongoing expanded access protocol (EAP) was to provide RNS60 to people living with ALS who are ineligible for controlled clinical trials and to collect data on the safety and tolerability of dosing RNS60 via twice-daily nebulization rather than the previously studied daily nebulization with weekly intravenous administration.
Methods: Eligible participants (≥ 18 years old, diagnosed with ALS per investigator assessment, and ineligible for an ALS clinical trial testing RNS60) were treated with twice-daily nebulization of RNS60 at home. Safety was evaluated by the assessment of adverse events and routine safety labs.
Results: A total of 84 participants have been treated with RNS60 via nebulization twice daily for up to 48 months so far. The most common treatment-related adverse event was increased secretions [N = 27 (32%)]. Serious adverse events (SAEs) [69 occurrences; N = 38 (45%) with at least one SAE] and deaths [N = 24 (28%)] were deemed not related to RNS60.
Discussion: This EAP supports the benign side effect profile of RNS60 when administered via twice-daily nebulization and demonstrates the feasibility of long-term EAPs as a complementary approach to controlled trials in people with advanced ALS.
Keywords: RNS60; amyotrophic lateral sclerosis; expanded access; motor neuron disease; neuroinflammation.
© 2025 The Author(s). Muscle & Nerve published by Wiley Periodicals LLC.
Conflict of interest statement
Mr. Sherman reports research grants from the ALS Association, ALS Finding a Cure, and the National Institutes of Health. Mr. Mock is a full‐time employee of Revalesio. Dr. Kalmes is a full‐time employee of Revalesio. Dr. Babu has received research funding from the American Association of Neuromuscular & Electrodiagnostic Medicine Foundation, the American Academy of Neurology, the ALS Association, the Muscular Dystrophy Association, Biogen, Orion Pharma, Novartis, Medicinova, Ionis, and AI Therapeutics. Dr. Berry reports research grants from Biogen, MT Pharma of America, Alexion, Rapa Therapeutics, the ALS Association, the Muscular Dystrophy Association, ALS One, Tambourine, ALS Finding a Cure, and reports personal consulting fees from Biogen, Clene Nanomedicine, MT Pharma of America, and Janssen. Dr. Cudkowicz received an institutional grant from Revalasio. Dr. Paganoni reports research grants from Amylyx Therapeutics, Revalesio, Eledon, Alector, UCB Pharma, Biohaven, Clene Nanomedicine, Prilenia Therapeutics, Seelos, Calico, Denali, NIH, DoD, and the Muscular Dystrophy Association and reports consulting fees from Amylyx, Arrowhead, Biogen, BMS, Clene, Cytokinetics, Eikonizo, J&J, Merck, PharmAust, Prilenia, and Sola. She has been a paid educational speaker for Medscape, PeerView, and i3Health. The remaining authors declare no potential conflicts of interest.
Figures
References
-
- Johnson S. A., Fang T., de Marchi F., et al., “Pharmacotherapy for Amyotrophic Lateral Sclerosis: A Review of Approved and Upcoming Agents,” Drugs 82, no. 13 (2022): 1367–1388. - PubMed
-
- “Safety and Efficacy of Edaravone in Well Defined Patients With Amyotrophic Lateral Sclerosis: A Randomised, Double‐Blind, Placebo‐Controlled Trial,” Lancet Neurology 16, no. 7 (2017): 505–512. - PubMed
-
- Blair H. A., “Tofersen: First Approval,” Drugs 83, no. 11 (2023): 1039–1043. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous