Circulating Extracellular Vesicles as Putative Mediators of Cardiovascular Disease in Paediatric Chronic Kidney Disease
- PMID: 40116365
- PMCID: PMC11926757
- DOI: 10.1002/jev2.70062
Circulating Extracellular Vesicles as Putative Mediators of Cardiovascular Disease in Paediatric Chronic Kidney Disease
Abstract
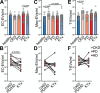
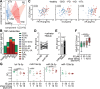
Cardiovascular disease (CVD) is the leading cause of mortality in chronic kidney disease (CKD). However, the pathogenesis of CVD in CKD remains incompletely understood. Endothelial extracellular vesicles (EC-EVs) have previously been associated with CVD. We hypothesized that CKD alters EV release and cargo, subsequently promoting vascular remodelling. We recruited 94 children with CKD, including patients after kidney transplantation and healthy donors, and performed EV phenotyping and functional EV analyses in the absence of age-related comorbidities. Plasma EC-EVs were increased in haemodialysis patients and decreased after kidney transplantation. Thirty microRNAs were less abundant in total CKD plasma EVs with predicted importance in angiogenesis and smooth muscle cell proliferation. In vitro, CKD plasma EVs induced transcriptomic changes in angiogenesis pathways and functionally impaired angiogenic properties, migration and proliferation in ECs. High shear stress, as generated by arterio-venous fistulas, and uremic toxins were considered as potential drivers of EV release, but only the combination increased EV generation from venous ECs. The resulting EVs recapitulated miRNA changes observed in CKD in vivo. In conclusion, CKD results in the release of EVs with altered miRNA profiles and anti-angiogenic properties, which may mediate vascular pathology in children with CKD. EVs and their miRNA cargo may represent future therapeutic targets to attenuate CVD in CKD.
Keywords: angiogenesis; cardiovascular disease; chronic kidney disease; microRNAs; shear stress; uremic toxins.
© 2025 The Author(s). Journal of Extracellular Vesicles published by Wiley Periodicals, LLC on behalf of the International Society for Extracellular Vesicles.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

