Efficient genetic manipulation of Shewanella through targeting defense islands
- PMID: 40116498
- PMCID: PMC12016545
- DOI: 10.1128/aem.02499-24
Efficient genetic manipulation of Shewanella through targeting defense islands
Abstract
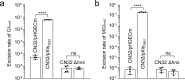
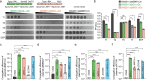
The Shewanella genus is widely recognized for its remarkable respiratory adaptability in anaerobic environments, exhibiting potential for bioremediation and microbial fuel cell applications. However, the genetic manipulation of certain Shewanella strains is hindered by defense systems that limit their genetic modification in biotechnology processes. In this study, we present a systematic method for predicting, mapping, and functionally analyzing defense islands within bacterial genomes. We investigated the genetically recalcitrant strain Shewanella putrefaciens CN32 and identified several defense systems located on two genomic islands integrated within the conserved chromosomal genes trmA and trmE. Our experimental assays demonstrated that overexpression of excisionases facilitated the excision of these islands from the chromosome, and their removal significantly enhanced the genetic manipulation efficiency of S. putrefaciens CN32. Further analysis revealed that these defense islands are widespread across various Shewanella strains and other gram-negative bacteria. This study presents an effective strategy to circumvent genetic barriers and fully exploit the potential of Shewanella for environmental and microbial engineering applications.
Importance: Efficiently modifying bacterial genomes is critical for advancing their industrial applications. However, bacteria in complex environments naturally develop defense mechanisms in response to bacteriophages and exogenous DNA, which pose significant challenges to their genetic modification. Several methods have emerged to tackle these challenges, including in vitro methylation of plasmid DNA and targeting specific restriction-modification (R-M) and CRISPR loci. Nevertheless, many bacteria harbor multiple, often uncharacterized defense mechanisms, limiting these strategies. Our study differs from previous approaches by specifically targeting defense islands-clusters of defense systems located within mobile genetic elements. Here, we investigated Shewanella putrefaciens CN32 and identified two key defense islands responsible for these protective functions. By selectively deleting these defense islands, we significantly enhanced the efficiency of genetic manipulation in S. putrefaciens. Our findings not only demonstrate a promising strategy for improving genetic engineering in Shewanella but also suggest broader applicability across other bacterial species. This work opens new opportunities for optimizing microbial processes in biotechnology, highlighting the potential of defense island-targeted genetic modification.
Keywords: Shewanella; Shewanella putrefaciens; defense system; genetic manipulation; genomic islands.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
FlrA Represses Transcription of the Biofilm-Associated bpfA Operon in Shewanella putrefaciens.Appl Environ Microbiol. 2017 Feb 1;83(4):e02410-16. doi: 10.1128/AEM.02410-16. Print 2017 Feb 15. Appl Environ Microbiol. 2017. PMID: 27986717 Free PMC article.
-
Sodium Lactate Negatively Regulates Shewanella putrefaciens CN32 Biofilm Formation via a Three-Component Regulatory System (LrbS-LrbA-LrbR).Appl Environ Microbiol. 2017 Jun 30;83(14):e00712-17. doi: 10.1128/AEM.00712-17. Print 2017 Jul 15. Appl Environ Microbiol. 2017. PMID: 28500045 Free PMC article.
-
Combined genomics and experimental analyses of respiratory characteristics of Shewanella putrefaciens W3-18-1.Appl Environ Microbiol. 2013 Sep;79(17):5250-7. doi: 10.1128/AEM.00619-13. Epub 2013 Jun 28. Appl Environ Microbiol. 2013. PMID: 23811511 Free PMC article.
-
Shewanella biofilm development and engineering for environmental and bioenergy applications.Curr Opin Chem Biol. 2020 Dec;59:84-92. doi: 10.1016/j.cbpa.2020.05.004. Epub 2020 Aug 1. Curr Opin Chem Biol. 2020. PMID: 32750675 Review.
-
[Opportunistic infections caused by Shewanella, new emergent bacteria].Med Mal Infect. 2005 Apr;35(4):186-91. doi: 10.1016/j.medmal.2005.03.008. Med Mal Infect. 2005. PMID: 15914286 Review. French.
References
-
- Fredrickson JK, Romine MF, Beliaev AS, Auchtung JM, Driscoll ME, Gardner TS, Nealson KH, Osterman AL, Pinchuk G, Reed JL, Rodionov DA, Rodrigues JLM, Saffarini DA, Serres MH, Spormann AM, Zhulin IB, Tiedje JM. 2008. Towards environmental systems biology of Shewanella. Nat Rev Microbiol 6:592–603. doi:10.1038/nrmicro1947 - DOI - PubMed
-
- Lies DP, Hernandez ME, Kappler A, Mielke RE, Gralnick JA, Newman DK. 2005. Shewanella oneidensis MR-1 uses overlapping pathways for iron reduction at a distance and by direct contact under conditions relevant for biofilms. Appl Environ Microbiol 71:4414–4426. doi:10.1128/AEM.71.8.4414-4426.2005 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- ONCE/Ocean Negative Carbon Emissions
- 42188102/National Science Foundation of China
- 2022YFC3103600/National Key Research and Development Program of China
- SCSIO2023QY03/Special fund of South China Sea Institute of Oceanology of the Chinese Academy of Sciences
- 2021345/Youth Innovation Promotion Association of the Chinese Academy of Sciences
LinkOut - more resources
Full Text Sources

