Synthetic Biology in Natural Product Biosynthesis
- PMID: 40116601
- PMCID: PMC12159196
- DOI: 10.1021/acs.chemrev.4c00567
Synthetic Biology in Natural Product Biosynthesis
Abstract
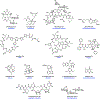
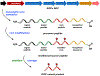
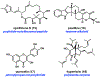
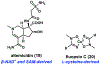
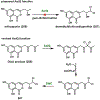
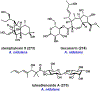
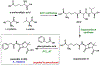
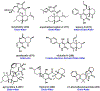
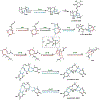
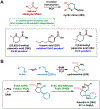
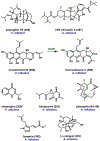
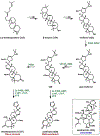
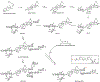
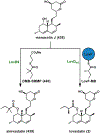
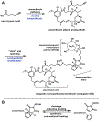
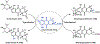
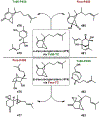
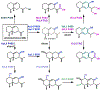
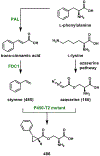
Synthetic biology has played an important role in the renaissance of natural products research during the post-genomics era. The development and integration of new tools have transformed the workflow of natural product discovery and engineering, generating multidisciplinary interest in the field. In this review, we summarize recent developments in natural product biosynthesis from three different aspects. First, advances in bioinformatics, experimental, and analytical tools to identify natural products associated with predicted biosynthetic gene clusters (BGCs) will be covered. This will be followed by an extensive review on the heterologous expression of natural products in bacterial, fungal and plant organisms. The native host-independent paradigm to natural product identification, pathway characterization, and enzyme discovery is where synthetic biology has played the most prominent role. Lastly, strategies to engineer biosynthetic pathways for structural diversification and complexity generation will be discussed, including recent advances in assembly-line megasynthase engineering, precursor-directed structural modification, and combinatorial biosynthesis.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


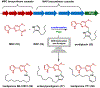
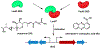
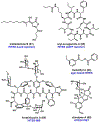
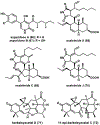
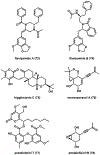
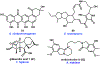
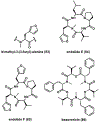
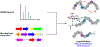





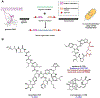

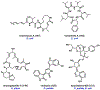

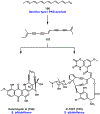


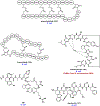








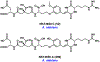

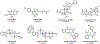




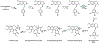
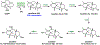
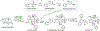
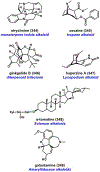
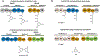

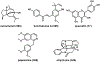
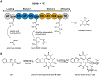
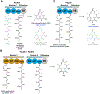

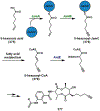

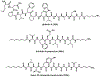
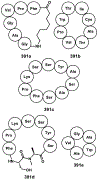
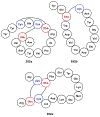
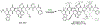

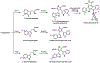
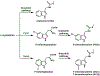

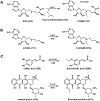
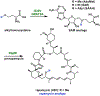

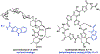



References
-
- Walsh CT; Tang Y Natural Product Biosynthesis; The Royal Society of Chemistry, 2022. 10.1039/9781837671069. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

