STIM1/2 maintain signaling competence at ER-PM contact sites during neutrophil spreading
- PMID: 40116769
- PMCID: PMC11927589
- DOI: 10.1083/jcb.202406053
STIM1/2 maintain signaling competence at ER-PM contact sites during neutrophil spreading
Abstract
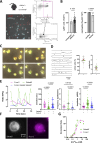
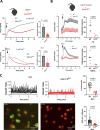
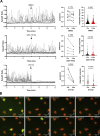
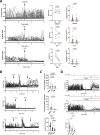
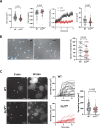
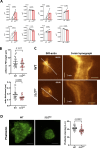
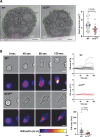
Neutrophils are highly motile leukocytes that migrate inside tissues to destroy invading pathogens. Ca2+ signals coordinate leukocytes migration, but whether Ca2+ fluxes mediated by Stim proteins at ER-PM contact sites regulate neutrophil actin-based motility is unclear. Here, we show that myeloid-specific Stim1/2 ablation decreases basal cytosolic Ca2+ levels and prevents adhesion-induced Ca2+ elevations in mouse neutrophils, reducing actin fiber formation and impairing spreading. Unexpectedly, more ER-PM contact sites were detected on the actin-poor adhesive membranes of Stim1/2-deficient neutrophils, which had reduced inositol-1,4,5-trisphosphate receptor (IP3R) immunoreactivity on confocal and immunogold micrographs despite preserved IP3R levels on western blots. Remarkably, Stim1/2-deficient neutrophils regained signaling and spreading competence in Ca2+-rich solutions and were recruited more effectively in mouse inflamed cremaster muscles in vivo. Our findings indicate that Stim1/2 preserve IP3R functionality in neutrophils, generating adhesion-dependent Ca2+ signals that control actin dynamics during neutrophil spreading. Stim proteins thus maintain IP3R signaling competence at adhesive membranes, enabling Ca2+-dependent actin remodeling during spreading in mouse neutrophils.
© 2025 Rabesahala de Meritens et al.
Conflict of interest statement
Disclosures: The authors declare no competing interests exist.
Figures







References
-
- Arige, V., Terry L.E., Wagner L.E. II, Malik S., Baker M.R., Fan G., Joseph S.K., Serysheva I.I., and Yule D.I.. 2022. Functional determination of calcium-binding sites required for the activation of inositol 1,4,5-trisphosphate receptors. Proc. Natl. Acad. Sci. USA. 119:e2209267119. 10.1073/pnas.2209267119 - DOI - PMC - PubMed
-
- Bengtsson, T., Jaconi M.E., Gustafson M., Magnusson K.E., Theler J.M., Lew D.P., and Stendahl O.. 1993. Actin dynamics in human neutrophils during adhesion and phagocytosis is controlled by changes in intracellular free calcium. Eur. J. Cell Biol. 62:49–58. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

