PRGN-2009 and bintrafusp alfa for patients with advanced or metastatic human papillomavirus-associated cancer
- PMID: 40116923
- PMCID: PMC11928712
- DOI: 10.1007/s00262-025-04009-z
PRGN-2009 and bintrafusp alfa for patients with advanced or metastatic human papillomavirus-associated cancer
Abstract
Background: This first-in-human phase 1 study (NCT04432597) evaluated the safety and recommended phase 2 dose (RP2D) of PRGN-2009, a gorilla adenoviral-vector targeting oncoproteins E6, E7 (human papillomavirus (HPV)16/18) and E5 (HPV16), as monotherapy (Arm 1A) and combined with the bifunctional TGF-β "trap"/anti-PD-L1 fusion protein bintrafusp alfa (BA; Arm 1B), in patients with recurrent/metastatic HPV-associated cancer.
Methods: Patients with ≥ 1 prior treatment (immunotherapy allowed) received PRGN-2009 (1 × 1011 particle units or 5 × 1011 particle units, subcutaneously) every 2 weeks for 3 doses, then every 4 weeks (Arm 1A), or PRGN-2009 (RP2D, schedule per Arm 1A) and BA (1200 mg, intravenously) every 2 weeks (Arm 1B). Primary endpoints were safety and RP2D of PRGN-2009; secondary objectives included overall response rate (ORR) and overall survival (OS).
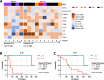
Results: Seventeen patients were treated. In Arm 1A (n = 6) there were no dose limiting toxicities or grade 3/4 treatment-related adverse events (TRAEs), 5 × 1011 PU was selected as RP2D, no responses were observed, and median OS (mOS) was 7.4 months (95% CI 2.9-26.8). In Arm 1B (n = 11), grade 3/4 TRAEs occurred in 27% of patients, ORR was 20% for all patients (22% in checkpoint-resistant patients), and mOS was 24.6 months (95% CI 9.6-not reached). Multifunctional HPV-specific T cells were increased or induced de novo in 80% of patients and not impacted by anti-vector antibodies. Higher serum IL-8 at baseline associated with shorter OS.
Conclusions: PRGN-2009 was well tolerated, and immune responses were observed to PRGN-2009. Encouraging anti-tumor activity and OS were noted in the combination with BA arm, consisting mainly of checkpoint-resistant patients. Trial Registration ClinicalTrials.gov Identifier: NCT04432597.
Keywords: Cervical cancer; Gene therapy; HPV; Immune checkpoint blockade; Oropharyngeal cancer; TGF-beta inhibition.
© 2025. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
Declarations. Conflict of interests: James L. Gulley and Julius Strauss are co-inventors on a patent for combination PDL1 and TGF-beta blockade in patients with HPV + malignancies. Amy Lankford and Douglas E. Brough are employees of Precigen, Inc., and hold stock ownership. Douglas E. Brough is a named inventor in patents pending. Ethics approval and consent to participate: This study was conducted in accordance with all applicable regulatory requirements. All protocols were approved by the Institutional Review Board of the Center for Cancer Research at the National Cancer Institute. All participants provided written informed consent.
Figures



References
-
- Centers for Disease Control and Prevention (2023) How many cancers are linked with HPV each year? https://www.cdc.gov/cancer/hpv/statistics/cases.htm. Accessed 26 Feb 2024
-
- Mashiana SS, Navale P, Khandakar B, Sobotka S, Posner MR et al (2021) Human papillomavirus genotype distribution in head and neck cancer: Informing developing strategies for cancer prevention, diagnosis, treatment and surveillance. Oral Oncol 113:105109. 10.1016/j.oraloncology.2020.105109 - PubMed
-
- Allen CT, Lewis JS Jr, El-Mofty SK, Haughey BH, Nussenbaum B (2010) Human papillomavirus and oropharynx cancer: biology, detection and clinical implications. Laryngoscope 120:1756–1772. 10.1002/lary.20936 - PubMed
-
- van der Burg SH, Melief CJ (2011) Therapeutic vaccination against human papilloma virus induced malignancies. Curr Opin Immunol 23:252–257. 10.1016/j.coi.2010.12.010 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

