Bifidobacterium lactis ameliorates AOM/DSS-induced inflammation, dysbiosis, and colonic precancerous lesions
- PMID: 40116950
- PMCID: PMC11928396
- DOI: 10.1007/s00253-025-13445-x
Bifidobacterium lactis ameliorates AOM/DSS-induced inflammation, dysbiosis, and colonic precancerous lesions
Abstract
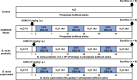
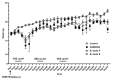
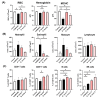
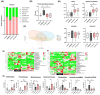
Bowel cancer is the third most common malignancy of tumors and one of the major causes of cancer-related death. Bowel precancerous conditions can develop without any symptoms, which either makes it difficult for early diagnosis or poses a poor prognosis/gloomy relapse. This study aimed to investigate the effects of Bifidobacterium animalis subsp. lactis TCI604 (B. lactis) on inflammatory responses, gut microbiome, and protectiveness against azoxymethane (AOM)/dextran sodium sulfate (DSS)-induced colonic precancerous lesions. The AOM/DSS-induced colonic precancerous lesion murine model was studied with 24 female C57BL/6 J mice assigned to the control group, AOM/DSS-induced colonic precancerous lesion group (AOM/DSS), AOM/DSS treated with B. lactis probiotic group (B. lactis P), and AOM/DSS treated with B. lactis cell-free supernatant group (B. lactis S). The results showed that both B. lactis P and B. lactis S could attenuate AOM/DSS-induced body weight loss and intestine damage, reduce aberrant crypt foci (ACF) and the formation of colonic polyps, and significantly inhibit pro-inflammatory cytokines and the NF-κB signaling pathway, in which the B. lactis S group outperformed others. Further analysis using 16S rDNA sequencing suggested that both B. lactis P and B. lactis S optimize gut microbiota. Several bacteria, including Muribaculaceae, Prevotellaceae_UCG-001, Anaerostipes, Ruminococcaceae, Mucispirillum, Clostridia_UCG-014, and Clostridia_vadinBB60 that were known in close relation to colonic precancerous lesions, were sequenced at taxonomic level. Our results indicated that both B. lactis P and B. lactis S improved AOM/DSS-induced colonic precancerous lesions by regulating inflammation as well as optimizing gut microbiota, thereby establishing reciprocally cooperative net benefits between probiotics/postbiotics and mice with colonic precancerous lesions. KEY POINTS: • Prophylactic administration of probiotic and postbiotic of B. lactis is capable of alleviating the AOM/DSS-induced body weight loss and colon shortening, as well as diminishing the development of colonic precancerous lesions, such as the formation of ACF and colonic polyps, in an AOM/DSS mouse model • Either probiotic or postbiotic of B. lactis has a positive role in mediating immune imbalance and colonic inflammation via suppression of inflammatory immune cells, pro-inflammatory cytokines, and the NF-κB signaling pathway • AOM/DSS-induced dysbiosis can be reversed with the probiotic and postbiotic of B. lactis supplementation.
Keywords: Bifidobacterium animalis subsp. Lactis; Colonic precancerous lesions; Gut microbiota; Postbiotics; Probiotics.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval: All protocols in this study were approved by the Institutional Animal Care and Use Committee (IACUC) at National Taiwan Ocean University (NTOU), Keelung, Taiwan (IACUC permit number: IACUC-110035), in compliance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health. Conflict of interest: The authors declare no competing interests.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

