Kinetic modelling reveals the presence of multistability in normal and stressful conditions in translational initiation mechanism
- PMID: 40117264
- PMCID: PMC11927921
- DOI: 10.1371/journal.pone.0319280
Kinetic modelling reveals the presence of multistability in normal and stressful conditions in translational initiation mechanism
Abstract
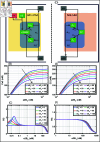
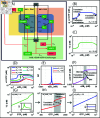
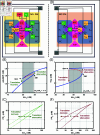
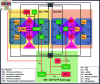
Protein synthesis involves translation initiation, elongation, termination, and ribosome recycling, and each step is controlled intricately by many signaling proteins. Translation initiation can be compactly categorized into two mechanisms: primary and secondary. The primary mechanism involves the recruitment of three important eukaryotic initiation factors, eIF2-GDP, eIF5, and eIF2B, and their interactions, followed by the GDP-GTP exchange by eIF2B to form an active dimer eIF2-GTP. The dimer binds with Met-tRNA to form a robust ternary complex (TC). The secondary mechanism closely mirrors the primary reaction mechanism, except that the interactions of eIF2B and eIF5 happen with the TC to form complexes. These interactions happen with high fidelity and precision, failing which fail-safe mechanisms are invoked instantaneously to delay the initiation process. In this work, we build a mathematical model to unravel how the transition between translation initiation and termination occurs at the initiation stage based on the elementary mechanisms we built from the network assembled from experimental observations. We focus only on the dynamics of primary and secondary mechanisms involved in the translation initiation process under normal and integrated stress response (ISR) conditions that act as a fail-safe mechanism by through phosphorylation-dephosphorylation (PdP) reactions. Since the network is huge and has many unknown kinetic parameters, we perform structural analysis using chemical reaction network theory (CRNT) and find hidden positive feedback loops that regulate the initiation mechanism. We apply bifurcation theory to show that the model exhibits ultrasensitivity and bistability under normal conditions, while under ISR, it exhibits both bistability and tristability for the choice of kinetic parameters. We attribute bistability to translation initiation and termination and tristability in ISR to translation recovery and attenuation. We conclude that the translation initiation process is a highly regulated process guided by the threshold and switching mechanisms to make quick decisions on the translation initiation, termination, recovery or attenuation under different conditions.
Copyright: © 2025 Harika, Sriram. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

