Suzetrigine, a Nonopioid Na V 1.8 Inhibitor for Treatment of Moderate-to-severe Acute Pain: Two Phase 3 Randomized Clinical Trials
- PMID: 40117446
- PMCID: PMC12061372
- DOI: 10.1097/ALN.0000000000005460
Suzetrigine, a Nonopioid Na V 1.8 Inhibitor for Treatment of Moderate-to-severe Acute Pain: Two Phase 3 Randomized Clinical Trials
Abstract
Background: Opioids are effective for treating acute pain but have safety, tolerability, and addiction concerns while nonopioid analgesics have limited efficacy. Suzetrigine, an oral, nonopioid small molecule, selectively inhibits the voltage-gated sodium channel 1.8 (NaV1.8) and has potential to provide efficacious and safe relief for acute pain without addiction concerns.
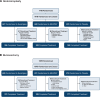
Methods: To evaluate suzetrigine for treatment of acute pain, two phase 3, randomized, double-blind, placebo- and active-controlled trials were conducted in adults with moderate-to-severe acute pain on the verbal categorical rating scale and 4 or greater on the numeric pain rating scale after abdominoplasty (n = 1,118) or bunionectomy (n = 1,073). After surgery, participants were randomized to suzetrigine (100 mg, then 50 mg every 12 h), hydrocodone bitartrate/acetaminophen (5/325 mg every 6 h), or placebo for 48 h. The primary endpoint was time-weighted sum of the pain intensity difference in numeric pain rating scale from 0 to 48 h (SPID48) versus placebo. Key secondary endpoints were SPID48 versus hydrocodone bitartrate/acetaminophen and time to 2-point or greater reduction in numeric pain rating scale from baseline versus placebo.
Results: The primary endpoint was achieved in both trials with suzetrigine demonstrating statistically significant and clinically meaningful reduction in pain versus placebo. The least squares mean difference in SPID48 between suzetrigine and placebo was 48.4 (95% CI, 33.6 to 63.1; P < 0.0001) after abdominoplasty and 29.3 (95% CI, 14.0 to 44.6; P = 0.0002) after bunionectomy. Neither trial achieved the first key secondary endpoint of superiority of suzetrigine versus hydrocodone bitartrate/acetaminophen on SPID48. For the second key secondary endpoint of time to 2-point or greater reduction in numeric pain rating scale, suzetrigine had a more rapid onset of clinically meaningful pain relief versus placebo after abdominoplasty (119 min vs. 480 min; nominal P < 0.0001) and bunionectomy (240 min vs. 480 min; nominal P = 0.0016). Adverse events were similar to those seen in postsurgical settings.
Conclusions: As compared with placebo, suzetrigine reduced moderate-to-severe acute pain over 48 h after abdominoplasty or bunionectomy. Pain reduction with suzetrigine was similar to that with hydrocodone bitartrate/acetaminophen. Suzetrigine was associated with adverse events that were mild to moderate in severity.
Copyright © 2025 The Author(s). Published by Wolters Kluwer Health, Inc., on behalf of the American Society of Anesthesiologists.
Conflict of interest statement
Drs. Bertoch, McCoun, Solanki, Taber, Urban, Oswald, Swisher, and Weiner have reported personal fees from Vertex Pharmaceuticals (Boston, Massachusetts) as members of the Acute Pain Steering Committee. Dr. Urban has reported personal fees and honoraria from Pacira Biosciences (Tampa, Florida). Dr. Swisher has reported fees or honoraria from Epimed International (Dallas, Texas), SPR Therapeutics (Cleveland, Ohio), InfuTronix (Natick, Massachusetts), Avanos Medical (Alpharetta, Georgia), Masimo (Irvine, California), and Varian Medical Systems (Palo Alto, California). Dr. Weiner has reported fees/honoraria from Cessation Therapeutics, Inc (Chapel Hill, North Carolina). Drs. Tian, Miao, Correll, Negulescu, and Bozic are employees of Vertex Pharmaceuticals and own stock and/or options in the company. Dr. D'Aunno reports no competing interests.
Figures


References
-
- Han C, Huang J, Waxman SG: Sodium channel Nav1.8: Emerging links to human disease. Neurology 2016; 86:473–83. doi:10.1212/WNL.0000000000002333 - PubMed
-
- Bennett DL, Clark AJ, Huang J, Waxman SG, Dib-Hajj SD: The role of voltage-gated sodium channels in pain signaling. Physiol Rev 2019; 99:1079–151. doi:10.1152/physrev.00052.2017 - PubMed
-
- Amaya F, Decosterd I, Samad TA, et al. : Diversity of expression of the sensory neuron-specific TTX-resistant voltage-gated sodium ion channels SNS and SNS2. Mol Cell Neurosci 2000; 15:331–42. doi:10.1006/mcne.1999.0828 - PubMed
-
- Akopian AN, Sivilotti L, Wood JN: A tetrodotoxin-resistant voltage-gated sodium channel expressed by sensory neurons. Nature 1996; 379:257–62. doi:10.1038/379257a0 - PubMed
-
- Renganathan M, Cummins TR, Waxman SG: Contribution of Nav1.8 sodium channels to action potential electrogenesis in DRG neurons. J Neurophysiol 2001; 86:629–40. doi:10.1152/jn.2001.86.2.629 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

