Risankizumab Versus Secukinumab: A Real-World Efficacy and Cost per Responder Comparison in Patients With Psoriasis in Italy
- PMID: 40117618
- PMCID: PMC11928122
- DOI: 10.5826/dpc.1501a4838
Risankizumab Versus Secukinumab: A Real-World Efficacy and Cost per Responder Comparison in Patients With Psoriasis in Italy
Abstract
Introduction: Risankizumab and secukinumab are effective treatment options for patients with moderate to severe psoriasis.
Objectives: We sought to estimate the efficacy and the cost per responder of risankizumab and secukinumab by comparing these two drugs in a real-life setting.
Methods: A multicentric retrospective study was conducted in patients from the Lazio region of Italy affected by moderate-to-severe psoriasis who initiated risankizumab or secukinumab between September 2020 to September 2022. Psoriasis Area and Severity Index (PASI) was measured at baseline and after 16, 52, and 78 weeks. Clinical responses were evaluated by PASI90 and PASI100 responses at the same timepoints. The cost per responder at week 16 and 52 was adopted as a cost-effectiveness indicator.
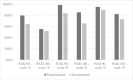
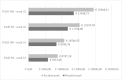
Results: Included were 141 patients, 74 (52.5%) treated with risankizumab and 67 (47.5%) treated with secukinumab. PASI90 responses in risankizumab-treated patients were higher than those observed in patients treated with secukinumab at both weeks 16 and 52 (79.7% versus 64.2% (P = 0.041) and 98.6% versus 83.6% (P = 0.003), respectively). Risankizumab also showed superior PASI100 rates at week 52 (85.5% versus 65.6%, P = 0.009). No statistically significant differences were observed in PASI90 and PASI100 rates between the 2 groups at week 78. The cost per PASI90 and PASI100 responder for risankizumab was lower at both weeks 16 (€5833.66 and €8394.78, compared to €8747.18 and €10746.53 for secukinumab) and 52 (€11798.90 and €13598.73 vs €15347.70 and €19568.31, respectively).
Conclusions: Risankizumab showed superior efficacy than secukinumab and a lower cost per responder.
Conflict of interest statement
Figures
References
-
- Armstrong AW, Read C. Pathophysiology, Clinical Presentation, and Treatment of Psoriasis: A Review. JAMA. 2020;323(19):1945–1960. - PubMed
-
- Alwan W, Nestle FO. Pathogenesis and treatment of psoriasis: exploiting pathophysiological pathways for precision medicine. Clin Exp Rheumatol. 2015;33(5 Suppl 93):S2–S6. - PubMed
LinkOut - more resources
Full Text Sources