Anti-PD1 prolongs the response of PI3K and farnesyl transferase inhibition in HRAS- and PIK3CA-mutant head and neck cancers
- PMID: 40117718
- PMCID: PMC11978339
- DOI: 10.1016/j.neo.2025.101157
Anti-PD1 prolongs the response of PI3K and farnesyl transferase inhibition in HRAS- and PIK3CA-mutant head and neck cancers
Abstract
Background: Tipifarnib, a farnesyl transferase inhibitor, has shown promising response in the treatment of HRAS-mutant HNSCC in the clinic, and in combination with a PI3K inhibitor in PIK3CA-mutant mouse models; however, the involvement of antitumor immunity in the efficacy of tipifarnib has not yet been investigated. This study aimed to evaluate the involvement of antitumor immunity in the efficacy of tipifarnib in HRAS- or PIK3CA-mutant HPV-positive and HPV-negative head and neck cancer murine models.
Methods: To investigate the role of antitumor immunity, we compared the efficacy of tipifarnib in immune-intact C57BL/6 mice and immunodeficient NSG mice. Histopathological analyses were conducted to evaluate PD-L1 expression and the activation of key signaling pathways. Additionally, the synergistic potential of tipifarnib with the PI3Kα inhibitor alpelisib (BYL719) was assessed in vitro and in vivo. Immunohistochemical analysis was performed to examine the infiltration of CD8+T cells, and anti-PD1 treatment was tested to evaluate its potential to prolong progression-free survival.
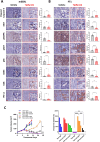
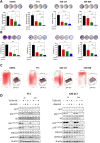
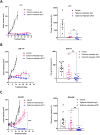
Results: In the HPV-positive HRAS-mutant HNSCC model, the antitumor efficacy of tipifarnib was primarily dependent on CD8+T cell activity, whereas in HPV-negative cancers, the contribution of antitumor immunity was less pronounced. Tipifarnib treatment upregulated PD-L1 expression, potentially inhibiting T cell antitumor activity and inducing hyperactivation of the AKT pathway, which mitigated MAPK inhibition and promoted cell proliferation. Blocking the PI3K pathway with alpelisib demonstrated synergistic antitumor effects in all models. The combination of tipifarnib and alpelisib exhibited greater efficacy in immune-intact mice than in immunodeficient mice, and was accompanied by increased CD8+T cell infiltration. Adding anti-PD1 treatment to the tipifarnib/alpelisib combination further prolonged progression-free survival in tumor-bearing mice.
Conclusion: These findings underscore the critical role of antitumor immunity, particularly CD8+T cell activity, in the efficacy of tipifarnib alone and in combination with alpelisib. The triple combination of tipifarnib, alpelisib, and anti-PD1 treatment showed superior antitumor activity and extended survival in preclinical models, suggesting its potential as a therapeutic strategy for HNSCC patients with HRAS- and PIK3CA-mutation.
Keywords: Alpelisib; Anti-PD1; HRAS; Head and neck cancer; PI3K; Tipifarnib.
Copyright © 2025. Published by Elsevier Inc.
Conflict of interest statement
Declaration of competing interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:Ben Gurion University of the Negev (investigator Prof. Moshe Elkabets) received a grant from Kura Oncology, Inc. Linda Kessler and Francis Burrows declare employment and stock or stock options with and travel expenses from Kura Oncology, Inc. This work was prepared while Gloria Su was employed at Columbia University Irving Medical Center. The opinions expressed in this article are the author's own and do not reflect the view of the National Institutes of Health, the Department of Health and Human Services, or the United States government. The remaining authors declare that they have no known competing financial interests or personal relationships that could influence the work reported in this study.
Figures




References
-
- Li P.C., Margalit D.N. Overview of head and neck cancer management. Improv. Therapeutic Ratio in Head Neck Cancer. 2020:1–32. doi: 10.1016/B978-0-12-817868-3.00001-9. - DOI
-
- Li H., Wawrose J.S., Gooding W.E., Garraway L.A., Lui V.W.Y., Peyser N.D., et al. Genomic analysis of head and neck squamous cell carcinoma cell lines and human tumors: a rational approach to preclinical model selection. Mol. Cancer Res. 2014;12:571–582. doi: 10.1158/1541-7786.MCR-13-0396/79751/AM/MOLECULAR-PROFILING-OF-HNSCC-CELLS-AND-TUMORS. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

