Influence of Surfactants with Differently Charged Headgroups on the Surface Propensity of Bromide
- PMID: 40118072
- PMCID: PMC11973919
- DOI: 10.1021/acs.jpca.4c07539
Influence of Surfactants with Differently Charged Headgroups on the Surface Propensity of Bromide
Abstract
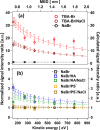
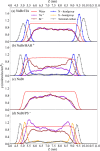
Halide ions in oceans and sea-spray aerosol particles are an important source of reactive halogen species in the atmosphere that impact the ozone budget and radiative balance. The multiphase cycling of halogen species is linked to the abundance of halide ions at the aqueous solution-air interface. Ubiquitously present surface-active organic compounds may affect the interfacial abundance of halide ions. Here, we use liquid jet X-ray photoelectron spectroscopy and molecular dynamics (MD) simulations to assess the impact of surfactants with different headgroups on the abundance of bromide and sodium ions at the interface. Core level spectra of Br 3d, Na 2s, and O 1s are reported for solutions containing tetrabutylammonium, hexylamine (HA), and propyl sulfate. We used a photoelectron attenuation model to retrieve the interfacial concentration of bromide in the presence of these different surfactants. The experimental results confirm the previously reported strong enhancement of bromide in the presence of tetrabutylammonium at the interface. In turn, propyl sulfate had a minor impact on the abundance of bromide but led to a significantly enhanced concentration of sodium cations. The MD simulations performed for bromide solutions containing hexylammonium and propyl sulfate show an enhancement of the interfacial bromide and sodium concentrations, respectively, comparable to the experimental results. The difference between the measured enhancement of bromide for HA and the nearly nonexistent effect of HA on bromide in the MD simulations is ascribed to the small amounts of hexylammonium present in the experimental solution. The present work suggests an important role of electrostatic interactions at the interface, which may guide the assessment of anion and cation abundances in atmospheric particles more generally.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Sander R.; Keene W. C.; Pszenny A. A. P.; Arimoto R.; Ayers G. P.; Baboukas E.; Cainey J. M.; Crutzen P. J.; Duce R. A.; Hönninger G.; et al. Inorganic bromine in the marine boundary layer: A critical review. Atmos. Chem. Phys. 2003, 3 (5), 1301–1336. 10.5194/acp-3-1301-2003. - DOI
-
- Wang S.; Schmidt J. A.; Baidar S.; Coburn S.; Dix B.; Koenig T. K.; Apel E.; Bowdalo D.; Campos T. L.; Eloranta E.; et al. Active and widespread halogen chemistry in the tropical and subtropical free troposphere. Proc. Natl. Acad. Sci. U.S.A. 2015, 112 (30), 9281–9286. 10.1073/pnas.1505142112. - DOI - PMC - PubMed
-
- Ghosal S.; Brown M. A.; Bluhm H.; Krisch M. J.; Salmeron M.; Jungwirth P.; Hemminger J. C. Ion partitioning at the liquid/vapor interface of a multicomponent alkali halide solution: A model for aqueous sea salt aerosols. J. Phys. Chem. A 2008, 112 (48), 12378–12384. 10.1021/jp805490f. - DOI - PubMed
-
- Artiglia L.; Edebeli J.; Orlando F.; Chen S.; Lee M.-T.; Corral Arroyo P.; Gilgen A.; Bartels-Rausch T.; Kleibert A.; Vazdar M.; et al. A surface-stabilized ozonide triggers bromide oxidation at the aqueous solution-vapour interface. Nat. Commun. 2017, 8 (1), 700. 10.1038/s41467-017-00823-x. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

