Positive airway pressure therapy and all-cause and cardiovascular mortality in people with obstructive sleep apnoea: a systematic review and meta-analysis of randomised controlled trials and confounder-adjusted, non-randomised controlled studies
- PMID: 40118084
- PMCID: PMC12045716
- DOI: 10.1016/S2213-2600(25)00002-5
Positive airway pressure therapy and all-cause and cardiovascular mortality in people with obstructive sleep apnoea: a systematic review and meta-analysis of randomised controlled trials and confounder-adjusted, non-randomised controlled studies
Abstract
Background: Data regarding the effect of positive airway pressure (PAP) therapy for obstructive sleep apnoea (OSA) on all-cause mortality are inconsistent. We aimed to conduct a systematic review and meta-analysis to test the hypothesis that PAP therapy is associated with reduced all-cause and cardiovascular mortality in people with OSA.
Methods: For this systematic review and meta-analysis, we searched PubMed, Embase, and the Cochrane Central Register of Controlled Trials, from database inception to Aug 22, 2023 (updated Sept 9, 2024), with no language or geographical restrictions. Reference lists of eligible studies and recent conference abstracts (2022-23) were also reviewed. We included outpatient studies (randomised controlled trials [RCTs] or confounder-adjusted, non-randomised controlled studies [NRCSs]) assessing the incidence of all-cause mortality, cardiovascular mortality, or both in adults (aged ≥18 years) with OSA who were treated versus not treated with PAP; other study types and studies that evaluated only PAP adherence were excluded. Abstracts of all retrieved publications were independently screened by two of three researchers (BS, SRB, and KCW), with disagreements resolved by adjudication from another researcher (SHM). The AutoLit feature of the Nested Knowledge platform was used for the review and data-extraction phases. We analysed each log-transformed hazard ratio (HR) and SE using a linear random-effects model to estimate overall HRs and 95% CIs. To evaluate the risk of bias, we used the Cochrane Risk of Bias tool for RCTs and the Newcastle-Ottawa Scale for NRCSs. This study was registered with PROSPERO, CRD42023456627.
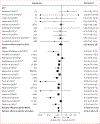
Findings: Of 5484 records identified by our search, 435 were assessed for eligibility and 30 studies were included in the systematic review and meta-analysis (ten RCTs and 20 NRCSs). These studies included 1 175 615 participants, of whom 905 224 (77%) were male and 270 391 (23%) were female (SE 1·9), with a mean age of 59·5 (SE 1·4) years and a mean follow-up of 5·1 (0·5) years. The risk of bias was low to moderate. The risk of all-cause mortality (HR 0·63, 95% CI 0·56-0·72; p<0·0001) and cardiovascular mortality (0·45, 0·29-0·72; p<0·0001) was significantly lower in the PAP group than in the no-PAP group, and the clinically relevant benefit of PAP therapy increased with use.
Interpretation: Our results are consistent with a potentially beneficial effect of PAP therapy on all-cause and cardiovascular mortality in patients with OSA. Patients should be made aware of this effect of their treatment, which could result in greater acceptance of treatment initiation and greater adherence, leading to a higher likelihood of improved outcomes.
Funding: ResMed.
Copyright © 2025 Elsevier Ltd. All rights reserved, including those for text and data mining, AI training, and similar technologies.
Conflict of interest statement
Declaration of interests AVB, AW, FL, and FHSK are employees of ResMed. J-LP is supported by the French National Research Agency in the framework of the Investissements d’Avenir programme (grant ANR-15-IDEX-02) and the e-Health and Integrated Care and Trajectories Medicine and MIAI Artificial Intelligence chairs of excellence from the Grenoble Alpes University Foundation (ANR-19-P3IA-0003); and reports lecture fees or conference travelling grants from ResMed, Philips, Jazz Pharmaceuticals, AGIR à dom, and Bioprojet. PAC reports an appointment to an endowed academic Chair at the University of Sydney that was established with ResMed funding; has received research support from ResMed and SomnoMed; and is a consultant to ResMed, SomnoMed, and Sunrise Medical. SHM, BS, SRB, and KCW are employees of Boston Strategic Partners, funded by ResMed. LW and CK are independent statisticians funded by ResMed. TK reports project funding from the Canadian Institute of Health Research and the CHEST Foundation. DAJ has received consultancy fees from Idorsia. RH has received speaker or consultancy fees from ResMed, Jazz Pharmaceuticals, Inspire Medical Systems, Bioprojet, Philips, Merck, Nyxoah, Medtronic, Nestlé, and Löwenstein. AM is funded by the National Institutes of Health and reports income related to medical education from LivaNova, Jazz Pharmaceuticals, ZOLL, and Eli Lilly. ResMed provided a philanthropic donation to University of California San Diego, but AM has not received personal income from ResMed or medXcloud. CHL declares no competing interests.
Figures

References
-
- Pepperell JC, Ramdassingh-Dow S, Crosthwaite N, et al. Ambulatory blood pressure after therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised parallel trial. Lancet 2002; 359: 204–10. - PubMed
-
- Jenkinson C, Davies RJ, Mullins R, Stradling JR. Comparison of therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised prospective parallel trial. Lancet 1999; 353: 2100–05. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

