Hepato-selective dihydroquinolizinones active against hepatitis A virus in vitro and in vivo
- PMID: 40118118
- PMCID: PMC12152923
- DOI: 10.1016/j.antiviral.2025.106145
Hepato-selective dihydroquinolizinones active against hepatitis A virus in vitro and in vivo
Abstract
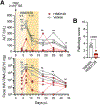
Despite the considerable clinical and economic burden imposed by hepatitis A virus (HAV) infection, both globally and in U.S., there are currently no available antiviral therapies for the treatment of type A hepatitis. Here we describe novel third-generation hepato-selective dihydroquinolizinones (HS-DHQs) with cellular uptake mediated by transport via hepatocyte-specific solute organic anion transporter family members 1B1 and 1B3 (OATP1B1-B3). The lead HS-DHQ compound, HS83128, demonstrates robust inhibition of the host cell TENT4A/B terminal nucleotidyltransferases required for efficient HAV RNA synthesis (IC50 6-25nM), and potent antiviral activity against HAV in cell culture (EC50 0.6 nM). Pharmacokinetic studies in CD-1 mice receiving comparable oral doses of HS83128 and a first-generation dihydroquinolizinone, RG7834, revealed a 5-fold increase in intrahepatic drug concentration and more than 10-fold improvement in liver versus nervous system tissue selectivity. Twice-daily oral administration of HS83128 rapidly arrested viral replication in HAV-infected Ifnar1-/- mice, reducing fecal virus shedding and cytokine markers of hepatic inflammation and reversing virus-induced liver injury. The hepato-selective nature of HS83128 may reduce the risk of neurologic and reproductive track toxicities observed with long-term administration of other dihydroquinolizinones, making it a candidate for the first antiviral therapy of hepatitis A.
Keywords: Antiviral drug; Hepatovirus; OATP1 transporter; PAPD poly(A) polymerase; Pharmacokinetics; Prophylaxis; TENT4 terminal nucleotidyltransferase; Therapy.
Published by Elsevier B.V.
Conflict of interest statement
Declaration of competing interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:Y. Du, Y. Li, T. Block and S.M. Lemon are listed as inventors on a patent application related to HS-DHQ antivirals for hepatitis A. Y. Du and T. Block have ownership interests in Harlingene Life Sciences.
Figures

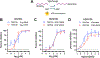
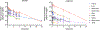


References
-
- Bopst M, Dinklo T, Funk J, Greiter-Wilke A, Lenz B, Kustermann S, Jiang T, Xie J, 2023. Unexpected neurotoxicity in chronic toxicity studies with a HBV viral expression inhibitor. Regul. Toxicol. Pharmacol. 141, 105407. - PubMed
-
- Evers R, Chu XY, 2008. Role of the murine organic anion-transporting polypeptide 1b2 (Oatp1b2) in drug disposition and hepatotoxicity. Mol. Pharmacol. 74, 309–311. - PubMed
-
- Glikson M, Galun E, Oren R, Tur-Kaspa R, Shouval D, 1992. Relapsing hepatitis A. Review of 14 cases and literature survey. Medicine (Baltim.) 71, 14–23. - PubMed
-
- Gotchev D, Dorsey BD, Nguyen D, Kakarla R, Dugan B, Chen S, Gao M, Bailey L, Liu F, Harasym T, Chiu T, Tang S, Lee AC, Cole AG, Sofia MJ, 2024. Structure-activity relationships and discovery of (S)-6-Isopropyl-2-methoxy-3-(3-methoxypropoxy)-10-oxo-5,10-dihydro-6H-pyrido[1,2-h][1,7]naphthyridine-9-carboxylic acid (AB-452), a novel orally available HBV RNA destabilizer. J. Med. Chem. 67, 1421–1446. - PubMed
-
- Han X, Zhou C, Jiang M, Wang Y, Wang J, Cheng Z, Wang M, Liu Y, Liang C, Wang J, Wang Z, Weikert R, Lv W, Xie J, Yu X, Zhou X, Luangsay S, Shen HC, Mayweg AV, Javanbakht H, Yang S, 2018. Discovery of RG7834: the first-in-class selective and orally available small molecule hepatitis B virus expression inhibitor with novel mechanism of action. J. Med. Chem. 61, 10619–10634. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

