Cleavage of the TrkB-FL receptor during epileptogenesis: insights from a kainic acid-induced model of epilepsy and human samples
- PMID: 40118354
- PMCID: PMC12033085
- DOI: 10.1016/j.phrs.2025.107707
Cleavage of the TrkB-FL receptor during epileptogenesis: insights from a kainic acid-induced model of epilepsy and human samples
Abstract
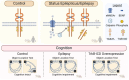
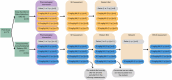
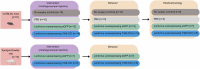
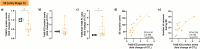
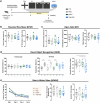
Brain-derived neurotrophic factor (BDNF) is essential for neuronal survival, differentiation, and plasticity. In epilepsy, BDNF exhibits a dual role, exerting both antiepileptic and pro-epileptic effects. The cleavage of its main receptor, full-length tropomyosin-related kinase B (TrkB-FL), was suggested to occur in status epilepticus (SE) in vitro. Moreover, under excitotoxic conditions, TrkB-FL was found to be cleaved, resulting in the formation of a new intracellular fragment, TrkB-ICD. Thus, we hypothesized that TrkB-FL cleavage and TrkB-ICD formation could represent an uncovered mechanism in epilepsy. We used a rat model of mesial temporal lobe epilepsy (mTLE) induced by kainic acid (KA) to investigate TrkB-FL cleavage and TrkB-ICD formation during SE (∼3 h after KA) and established epilepsy (EE) (4-5 weeks after KA). Animals treated with 10 mg/kg of KA exhibited TrkB-FL cleavage during SE, with hippocampal levels of TrkB-FL and TrkB-ICD correlating with seizure severity. Notably, TrkB-FL cleavage and TrkB-ICD formation were also detected in animals with EE, which exhibited spontaneous recurrent convulsive seizures, neuronal death, mossy fiber sprouting, and long-term memory impairment. Importantly, hippocampal samples from patients with refractory epilepsy also showed TrkB-FL cleavage with increased TrkB-ICD levels. Additionally, lentiviral-mediated overexpression of TrkB-ICD in the hippocampus of healthy mice and rats resulted in long-term memory impairment. Our findings suggest that TrkB-FL cleavage and the subsequent TrkB-ICD production occur throughout epileptogenesis, with the extent of cleavage correlating positively with seizure occurrence. Moreover, we found that TrkB-ICD overexpression impairs memory. This work uncovers a novel mechanism in epileptogenesis that could serve as a potential therapeutic target in mTLE, with implications for preserving cognitive function.
Keywords: BDNF; Cognition; Spontaneous seizures; Status epilepticus; TrkB-ICD.
Copyright © 2025 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: João Fonseca-Gomes has patent #PCT/PT2021/050011 pending to Assignee. Maria Jose Diógenes has patent #PCT/PT2021/050011 pending to Assignee. Ana Maria Sebastião has patent #PCT/PT2021/050011 pending to Assignee. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




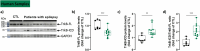


References
-
- Miranda-Lourenço* C., Ribeiro-Rodrigues* L., Fonseca-Gomes* J., Tanqueiro* S.R., Belo* R.F., Ferreira C.B., et al. Challenges of BDNF-based therapies: from common to rare diseases. Pharm. Res. 2020;162:105281. - PubMed
-
- Hou X., Wang X., Zhang L. Conditional downregulation of brain- derived neurotrophic factor and tyrosine kinase receptor B blocks epileptogenesis in the human temporal lobe epilepsy hippocampus. Neurol. India. 2010;58(1):29. - PubMed
-
- Takahashi M., Hayashi S., Kakita A., Wakabayashi K., Fukuda M., Kameyama S., et al. Patients with temporal lobe epilepsy show an increase in brain-derived neurotrophic factor protein and its correlation with neuropeptide Y. Brain Res. 1999;818:579–582. - PubMed
-
- Rudge J.S., Mather P.E., Pasnikowski E.M., Cai N., Corcoran T., Acheson A., et al. Endogenous BDNF protein is increased in adult rat hippocampus after a kainic acid induced excitotoxic insult but exogenous BDNF is not neuroprotective. Exp. Neurol. 1998;149:398–410. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

