Decreased Extracellular Vesicle Vasorin in Severe Preeclampsia Plasma Mediates Endothelial Dysfunction
- PMID: 40118804
- PMCID: PMC12132852
- DOI: 10.1161/JAHA.124.037242
Decreased Extracellular Vesicle Vasorin in Severe Preeclampsia Plasma Mediates Endothelial Dysfunction
Abstract
Background: Preeclampsia is a serious pregnancy complication affecting 5% to 8% of pregnancies globally. preeclampsia is a leading cause of maternal and neonatal morbidity and death. Despite its prevalence, the underlying mechanisms of preeclampsia remain unclear. This study investigated the role of vasorin in preeclampsia pathogenesis by examining its levels in extracellular vesicles (EVs) and effects on vascular function.
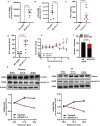
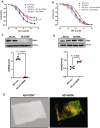
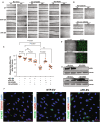
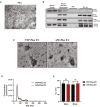
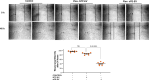
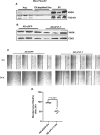
Methods and results: We conducted unbiased proteomics on urine-derived EVs from women with severe preeclampsia and normotensive pregnancies, identifying differentially abundant proteins. Vasorin expression levels were measured in urinary EVs, plasma EVs, and placental tissue. EVs were generated from human and murine placental explants. Vascular functions were assessed using murine aortic rings and human aortic endothelial cells. Vasorin expression was manipulated in human aortic endothelial cells via overexpression and knockdown followed by RNA sequencing. One hundred twenty proteins showed ≥±1.5-fold regulation (P<0.05) between severe preeclampsia and NTP. Vasorin levels decreased in severe preeclampsia in urinary EVs, plasma EVs, and placental tissue. Vasorin levels increased with gestational age in murine pregnancy and were diminished in a murine model of preeclampsia. Severe preeclampsia and murine preeclampsia EVs impaired human aortic endothelial cell migration and inhibited murine aortic ring vasorelaxation. Vasorin overexpression counteracted these effects. RNA sequencing showed that vasorin manipulation in human aortic endothelial cells differentially regulated hundreds of genes linked to vasculogenesis, proliferation, migration, and apoptosis.
Conclusions: The data suggest that vasorin, delivered to the endothelium via EVs, regulates vascular function and that the loss of EV vasorin may be one of the mechanistic drivers of preeclampsia.
Keywords: extracellular vesicles; human aortic endothelial cells; placental explant culture; preeclampsia; short FMS‐like tyrosine kinase 1; vasorin.
Conflict of interest statement
None.
Figures




Update of
-
Decreased Extracellular Vesicle Vasorin in Severe Preeclampsia Plasma Mediates Endothelial Dysfunction.bioRxiv [Preprint]. 2024 Jun 25:2024.06.24.600441. doi: 10.1101/2024.06.24.600441. bioRxiv. 2024. Update in: J Am Heart Assoc. 2025 Apr;14(7):e037242. doi: 10.1161/JAHA.124.037242. PMID: 38979275 Free PMC article. Updated. Preprint.
References
-
- Grandi SM, Filion KB, Yoon S, Ayele HT, Doyle CM, Hutcheon JA, Smith GN, Gore GC, Ray JG, Nerenberg K, et al. Cardiovascular disease‐related morbidity and mortality in women with a history of pregnancy complications. Circulation. 2019;139:1069–1079. doi: 10.1161/CIRCULATIONAHA.118.036748 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

