ANXA11 biomolecular condensates facilitate protein-lipid phase coupling on lysosomal membranes
- PMID: 40118863
- PMCID: PMC11928461
- DOI: 10.1038/s41467-025-58142-5
ANXA11 biomolecular condensates facilitate protein-lipid phase coupling on lysosomal membranes
Abstract
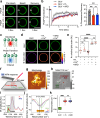
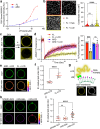
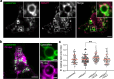
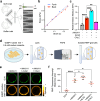
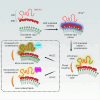
Phase transitions of cellular proteins and lipids play a key role in governing the organisation and coordination of intracellular biology. Recent work has raised the intriguing prospect that phase transitions in proteins and lipids can be co-regulated. Here we investigate this possibility in the ribonucleoprotein (RNP) granule-ANXA11-lysosome ensemble, where ANXA11 tethers RNP granules to lysosomal membranes to enable their co-trafficking. We show that changes to the protein phase state within this system, driven by the low complexity ANXA11 N-terminus, induces a coupled phase state change in the lipids of the underlying membrane. We identify the ANXA11 interacting proteins ALG2 and CALC as potent regulators of ANXA11-based phase coupling and demonstrate their influence on the nanomechanical properties of the ANXA11-lysosome ensemble and its capacity to engage RNP granules. The phenomenon of protein-lipid phase coupling we observe within this system serves as a potential regulatory mechanism in RNA trafficking and offers an important template to understand other examples across the cell whereby biomolecular condensates closely juxtapose organellar membranes.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: Tuomas Knowles and Peter St George-Hyslop are co-founders of TransitionBio. Jonathon Nixon-Abell and Seema Qamar are consultants in TransitionBio. TransitionBio has no involvement in the work described in this paper, but has an interest in biomolecular condensates in cancer and infectious disease. The remaining authors declare no competing interests.
Figures


Update of
-
ANXA11 biomolecular condensates facilitate protein-lipid phase coupling on lysosomal membranes.bioRxiv [Preprint]. 2023 Mar 24:2023.03.22.533832. doi: 10.1101/2023.03.22.533832. bioRxiv. 2023. Update in: Nat Commun. 2025 Mar 21;16(1):2814. doi: 10.1038/s41467-025-58142-5. PMID: 36993242 Free PMC article. Updated. Preprint.
References
-
- Eggeling, C. et al. Direct observation of the nanoscale dynamics of membrane lipids in a living cell. Nature457, 1159–1162 (2009). - PubMed
-
- Shaikh, S. R., Dumaual, A. C., Jenski, L. J. & Stillwell, W. Lipid phase separation in phospholipid bilayers and monolayers modeling the plasma membrane. Biochim. Biophys. Acta - Biomembr.1512, 317–328 (2001). - PubMed
-
- Simons, K. & Ikonen, E. Functional rafts in cell membranes. Nature387, 569–572 (1997). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

