Intratracheal Candida administration induced lung dysbiosis, activated neutrophils, and worsened lung hemorrhage in pristane-induced lupus mice
- PMID: 40118938
- PMCID: PMC11928548
- DOI: 10.1038/s41598-025-94632-8
Intratracheal Candida administration induced lung dysbiosis, activated neutrophils, and worsened lung hemorrhage in pristane-induced lupus mice
Abstract
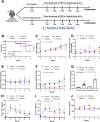
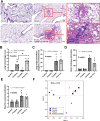
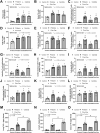
Because the innate immunity might and fungi in the lungs might enhance the severity of lupus-induced diffuse alveolar hemorrhage (DAH), intraperitoneal pristane injection was performed in C57BL6 mice with intratracheal administration by Candida albicans or phosphate buffer solution (PBS). Despite the similar pristane-induced lupus (proteinuria, serum creatinine, and serum anti-dsDNA) at 5 weeks of the model, Candida administration worsened several characteristics, including mortality, body weight, serum cytokines (TNF-α and IL-6), and lung hemorrhage score, and cytokines in the lung tissue (TNF-α, IL-6, and IL-10), but not gut permeability (FITC-dextran assay), serum IL-10, immune cells in the spleens (flow cytometry analysis), and activities of peritoneal macrophages (polymerase-chain reaction). Although Candida administration reduced proteobacterial abundance and altered alpha and beta diversity compared with PBS control, lung microbiota was not different between Candida administration in pristane- and non-pristane-administered mice. Because of the prominent Gram-negative bacteria in lung microbiota and the role of neutrophils in DAH, lipopolysaccharide (LPS) with and without heat-killed Candida preparation was tested. Indeed, Candida preparation with LPS induced more severe pro-inflammatory neutrophils than LPS stimulation alone as indicated by the expression of several genes (TNF-α, IL-6, IL-1β, IL-10, Dectin-1, and NF-κB). In conclusion, the intratracheal Candida worsened pristane-induced lung hemorrhage partly through the enhanced neutrophil responses against bacteria and fungi. More studies on Candida colonization in sputum from patients with lupus-induced DAH are interesting.
Keywords: Fungi; Lung hemorrhage; Lupus; Microbiome; Pristane.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures



References
-
- Bhunyakarnjanarat, T. et al. Lupus exacerbation in ovalbumin-induced asthma in Fc gamma receptor IIb deficient mice, partly due to hyperfunction of dendritic cells. Asian Pac. J. Allergy Immunol.10.12932/ap-290823-1677 (2024). - PubMed
-
- Chancharoenthana, W. et al. Enhanced lupus progression in alcohol-administered Fc gamma receptor-IIb-deficiency lupus mice, partly through leaky gut-induced inflammation. Immunol. Cell. Biol.101, 746–765. 10.1111/imcb.12675 (2023). - PubMed
-
- Pego-Reigosa, J. M., Medeiros, D. A. & Isenberg, D. A. Respiratory manifestations of systemic lupus erythematosus: Old and new concepts. Best Pract. Res. Clin. Rheumatol.23, 469–480. 10.1016/j.berh.2009.01.002 (2009). - PubMed
-
- Zamora, M. R., Warner, M. L., Tuder, R. & Schwarz, M. I. Diffuse alveolar hemorrhage and systemic lupus erythematosus. Clinical presentation, histology, survival, and outcome. Medicine76, 192–202. 10.1097/00005792-199705000-00005 (1997). - PubMed
-
- Quadrelli, S. A. et al. Pulmonary involvement of systemic lupus erythematosus: Analysis of 90 necropsies. Lupus18, 1053–1060. 10.1177/0961203309106601 (2009). - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
- grant No 272/2567/University of Phayao and Thailand Science Research and Innovation Fund, Fundamental Fund 2024
- B16F640175 and B48G660112/The Program Management Unit for Human Resources and Institutional Development, Research and Innovation
- RA-MF-22/65, RA-MF-13/66, and RA-MF-eAsia/Rachadapisek Sompote Matching Fund
- HEAF67300087/Thailand Science research and Innovation Fund Chulalongkorn University
- N41A640076/The National Research Council of Thailand (NRCT)
LinkOut - more resources
Full Text Sources
Medical

