SARS-Cov-2 vaccination strategies in hospitalized recovered COVID-19 patients: a randomized clinical trial (VATICO Trial)
- PMID: 40118946
- PMCID: PMC11932324
- DOI: 10.1038/s41598-025-92742-x
SARS-Cov-2 vaccination strategies in hospitalized recovered COVID-19 patients: a randomized clinical trial (VATICO Trial)
Abstract
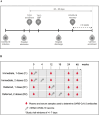
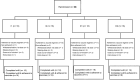
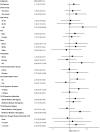
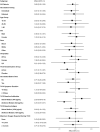
The impact on immunogenicity and efficacy of SARS-CoV-2 vaccination in people with prior COVID-19 could differ depending on timing of vaccination and number of doses. The VATICO study randomized 66 hospitalized recovered COVID-19 individuals to receive either immediate or deferred vaccination, with one or two doses of mRNA SARS-CoV-2 vaccines. We measured binding and neutralizing antibodies against SARS-CoV-2 at enrollment and longitudinally. Median (IQR) time from SARS-CoV-2 infection to first vaccination was 68 (53-75) days in the immediate group, and 151 (137-173) days in the deferred group. At week 48, timing or number of vaccine doses did not influence the change in antibody levels relative to baseline. Adherence to the assigned vaccine regimen was lower in the deferred group, particularly in participants receiving two doses. Although the study ultimately lacked adequate power to draw firm conclusions, these results suggest possible benefits of prompt vaccination after recovery from COVID-19.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: B.M., reports consultancy, advisory and/or speaker fees from AELIX Therapeutics, Gilead Sciences, AbbVie, Janssen, ViiV and MSD. R.P., has served on advisory boards for Gilead Sciences Inc., Pfizer Inc., Roche Therapeutics, MSD, GSK, ViiV Healthcare, Eli Lilly and Company, Astra Zeneca, Exevir, PharmaMar and Atea Pharmaceuticals Inc. He has had research grants paid to his institution by MSD, ViiV Healthcare, Gilead Sciences, and PharmaMar. M.K.J., has received research funding directed to her institution from Gilead Sciences, AbbVie, and Laurent. D.L.B., received money for advisory boards, lectures and travel grants paid to himself from the companies Gilead, MSD, ViiV and Pfizer. K.K., has received research funding from NIH, Astra Zeneca, Pfizer, Abbott, Romark, MSD, and Novartis and has served on advisory boards for the Burroughs Wellcome Fund, ParaFRAP, and the Sanford Guide. P.C.T., has received a grant support from Merck which was paid to her institution. G.V.M., have served on advisory board for Astra Zeneca. A.G., is named as an inventor on a patent covering a promoter construct used in ChAdOx1-vectored vaccines, including ChAdOx1 nCoV-19 vaccine, and has received royalty income through the University of Oxford’s from sales of Astra Zeneca vaccines and its sublicensees under the University’s revenue sharing policy whith potential benefits in the future. AG has also collaborated with Moderna and Novavax through her organization on commercial vaccine projects, which provided funding directly to the trials and the organization but not to her personally. J.M., has received research funding, consultancy fees and lecture sponsorships from and have served on advisory boards for MSD, Gilead Sciences, ViiV Healthcare, and Johnson & Johnson. The other authors declare non-financial competing interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

