H105A peptide eye drops promote photoreceptor survival in murine and human models of retinal degeneration
- PMID: 40118996
- PMCID: PMC11928584
- DOI: 10.1038/s43856-025-00789-8
H105A peptide eye drops promote photoreceptor survival in murine and human models of retinal degeneration
Abstract
Background: Photoreceptor death leads to inherited blinding retinal diseases, such as retinitis pigmentosa (RP). As disease progression often outpaces therapeutic advances, developing effective treatments is urgent. This study evaluates the efficacy of small peptides derived from pigment epithelium-derived factor (PEDF), which are known to restrict common cell death pathways associated with retinal diseases.
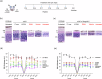
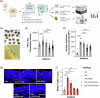
Methods: We tested chemically synthesized peptides (17-mer and H105A) with affinity for the PEDF receptor, PEDF-R, delivered as eye drops to two RP mouse models: rd10 (phosphodiesterase 6b mutation) and RhoP23H/+ (rhodopsin P23H mutation). Additionally, we engineered AAV-H105A vectors for intravitreal delivery in RhoP23H/+ mice. To assess peptide effects in human tissue, we used retinal organoids exposed to cigarette smoke extract, a model of oxidative stress. Photoreceptor survival, morphology and function were evaluated.
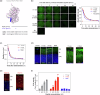
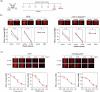
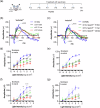
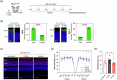
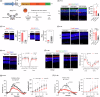
Results: Here we show that peptides 17-mer and H105A delivered via eye drops successfully reach the retina, promote photoreceptor survival, and improve retinal function in both RP mouse models. Intravitreal delivery of a AAV-H105A vector delays photoreceptor degeneration in RhoP23H/+ mice up to six months. In human retinal organoids, peptide H105A specifically prevents photoreceptor death induced by oxidative stress, a contributing factor to RP progression.
Conclusions: PEDF peptide-based eye drops offer a promising, minimally invasive therapy to prevent photoreceptor degeneration in retinal disorders, with a favorable safety profile.
Plain language summary
Retinitis pigmentosa (RP) is a rare inherited condition that causes the gradual death of photoreceptors (light-sensing cells) in the eye, leading to vision loss. There is currently no cure. This study tested a potential treatment using small protein fragments (peptides) from PEDF, a protective protein naturally found in the eye. Researchers delivered these peptides through eye drops or gene therapy in mouse models of RP and to human retinal organoids (lab-grown retina tissue). Mice treated early maintained healthy vision cells, while untreated mice experienced rapid cell loss and vision decline. These results suggest that peptide-based eye drops could be a simple, safe, and effective way to slow vision loss in patients with RP.
© 2025. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
Competing interests: US Government and University of Modena and Reggio Emilia, Italy. S. P. Becerra, A. Bernardo-Colón, V. Marigo, A Bighinati. US Application No. 63 430 251 NIH Ref No E-028-2023-0-US-01 and international application PCT/US2023/064947, filed on March 24, 2023. The specific aspect of the manuscript covered in the patent applications is on PIGMENT EPITHELIUM-DERIVED FACTOR PEPTIDES AND USE FOR TREATING RETINAL DEGENERATION.
Figures

Update of
-
H105A peptide eye drops promote photoreceptor survival in murine and human models of retinal degeneration.bioRxiv [Preprint]. 2024 Jul 16:2024.07.10.602890. doi: 10.1101/2024.07.10.602890. bioRxiv. 2024. Update in: Commun Med (Lond). 2025 Mar 21;5(1):81. doi: 10.1038/s43856-025-00789-8. PMID: 39109177 Free PMC article. Updated. Preprint.
References
-
- Hartong, D. T., Berson, E. L. & Dryja, T. P. Retinitis pigmentosa. Lancet368, 1795–1809 (2006). - PubMed
-
- T. B. O’Neal, E. E. Luther, in StatPearls. (Treasure Island (FL), 2024).
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

