Acute LPS exposure enhances susceptibility to peripheral prion infection
- PMID: 40119036
- PMCID: PMC11928655
- DOI: 10.1038/s41598-025-94003-3
Acute LPS exposure enhances susceptibility to peripheral prion infection
Abstract
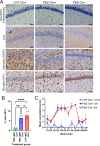
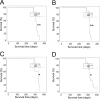
After peripheral infections, the initial accumulation of prions within secondary lymphoid tissues is essential for the transmission of disease to the brain. Macrophages are considered to sequester or destroy prions, but little was known of their impact on disease susceptibility after a peripheral infection. Inflammation in the peritoneal cavity can trigger the macrophage disappearance reaction, whereby the macrophages are temporarily contained within cellular aggregates on the mesothelium. We studied the impact of the bacterial lipopolysaccharide (LPS)-mediated macrophage disappearance reaction on susceptibility to an intraperitoneal prion infection. Intraperitoneal LPS injection significantly enhanced prion disease susceptibility approximately 100X when given 24-3 h before infection. The effects on disease susceptibility coincided with the reduced abundance of macrophages within the peritoneal cavity at the time of infection and the enhanced early accumulation of prions in the spleen. This suggests that the reduced recoverable abundance of macrophages in the peritoneal cavity following acute LPS-treatment, increased disease susceptibility by enhancing the initial propagation of the prions from site of exposure (peritoneal cavity) to the spleen from where they subsequently spread to the brain. Further studies may help identify novel macrophage-targeted treatments that can reduce susceptibility to peripherally acquired prion infections.
Keywords: Disease susceptibility; LPS; Macrophage; Peritoneal cavity; Prion diseases; Spleen; Transmissible spongiform encephalopathies.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures




References
-
- Legname, G. et al. Synthetic mammalian prions. Science305, 673–676. 10.1126/science.1100195 (2004). - PubMed
-
- Sigurdson, C. J. et al. Oral transmission and early lymphoid tropism of chronic wasting disease PrPres in mule deer fawns (Odocoileus hemionus). J. Gen. Virol.80, 2757–2764. 10.1099/0022-1317-80-10-2757 (1999). - PubMed
-
- Brown, K. L. et al. Scrapie replication in lymphoid tissues depends on PrP-expressing follicular dendritic cells. Nat. Med.5, 1308–1312. 10.1038/15264 (1999). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

