Evolutionary measures show that recurrence of DCIS is distinct from progression to breast cancer
- PMID: 40119428
- PMCID: PMC11929273
- DOI: 10.1186/s13058-025-01966-2
Evolutionary measures show that recurrence of DCIS is distinct from progression to breast cancer
Abstract
Background: Progression from pre-cancers like ductal carcinoma in situ (DCIS) to invasive disease (cancer) is driven by somatic evolution and is altered by clinical interventions. We hypothesized that genetic and/or phenotypic intra-tumor heterogeneity would predict clinical outcomes for DCIS since it serves as the substrate for natural selection among cells.
Methods: We profiled two samples from two geographically distinct foci from each DCIS in both cross-sectional (n = 119) and longitudinal cohorts (n = 224), with whole exome sequencing, low-pass whole genome sequencing, and a panel of immunohistochemical markers.
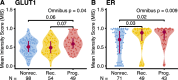
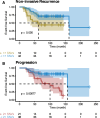
Results: In the longitudinal cohorts, the only statistically significant associations with time to non-invasive DCIS recurrence were the combination of treatment (lumpectomy only vs mastectomy or lumpectomy with radiation, HR 12.13, p = 0.003, Wald test with FDR correction), ER status (HR 0.16 for ER+ compared to ER-, p = 0.0045), and divergence in SNVs between the two samples (HR 1.33 per 10% divergence, p = 0.018). SNV divergence also distinguished between pure DCIS and DCIS synchronous with invasive disease in the cross-sectional cohort. In contrast, the only statistically significant associations with time to progression to invasive disease were the combination of the width of the surgical margin (HR 0.67 per mm, p = 0.043) and the number of mutations that were detectable at high allele frequencies (HR 1.30 per 10 SNVs, p = 0.02). No predictors were significantly associated with both DCIS recurrence and progression to invasive disease, suggesting that the evolutionary scenarios that lead to these clinical outcomes are markedly different.
Conclusions: These results imply that recurrence with DCIS is a clinical and biological process different from invasive progression.
Keywords: Breast cancer; Copy number alterations; Ductal carcinoma in situ; Evolutionary biomarkers; Intratumor heterogeneity; Invasive ductal carcinoma; Progression; Recurrence; Single nucleotide variants; Tumor evolution.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The Institutional Review Board (IRB) of Duke University Medical Center, as well as the IRB at each participating institution, approved this study, and a waiver of consent was obtained according to the approved protocol. Consent for publication: Not applicable. Competing interests: CC serves on the Scientific Advisory Board and/or as consultant for Bristol Myers Squibb, Deepcell, Genentech, NanoString, Ravel, Viosera, and holds equity in Deepcell, Illumina/Grail, and Ravel.
Figures




Update of
-
Evolutionary Measures Show that Recurrence of DCIS is Distinct from Progression to Breast Cancer.medRxiv [Preprint]. 2024 Aug 16:2024.08.15.24311949. doi: 10.1101/2024.08.15.24311949. medRxiv. 2024. Update in: Breast Cancer Res. 2025 Mar 21;27(1):43. doi: 10.1186/s13058-025-01966-2. PMID: 39185534 Free PMC article. Updated. Preprint.
References
-
- Rosenberg RD, Seidenwurm D. Optimizing breast cancer screening programs: experience and structures. Radiology. 2019;292:297–8. - PubMed
-
- Ozanne EM, Shieh Y, Barnes J, Bouzan C, Hwang ES, Esserman LJ. Characterizing the impact of 25 years of DCIS treatment. Breast Cancer Res Treat. 2011;129:165–73. - PubMed
-
- Maxwell AJ, Clements K, Hilton B, Dodwell DJ, Evans A, Kearins O, et al. Risk factors for the development of invasive cancer in unresected ductal carcinoma in situ. Eur J Surg Oncol. 2018;44:429–35. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

