Curative timed NK cell-based immunochemotherapy aborts brain tumour recurrence driven by mesenchymal glioma stem cells
- PMID: 40119461
- PMCID: PMC11927124
- DOI: 10.1186/s40478-025-01984-3
Curative timed NK cell-based immunochemotherapy aborts brain tumour recurrence driven by mesenchymal glioma stem cells
Abstract
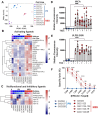
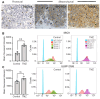
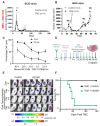
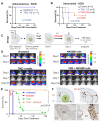
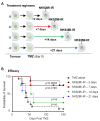
High grade gliomas (HGG) are incurable brain cancers, where inevitable disease recurrence is driven by tumour-initiating glioma stem cells (GSCs). GSCs survive and expand in the brain after surgery, radiation and temozolomide (TMZ) chemotherapy, amidst weak immune and natural killer (NK) cell surveillance. The present study was designed to understand how to enhance the contribution of innate immunity to post TMZ disease control. Strikingly, molecular subtypes of HGG impacted the repertoire of NK cell sensitivity markers across human HGG transcriptomes, and in a panel of GSCs with either proneural (PN-GSC) or mesenchymal (MES-GSC) phenotypes. Indeed, only MES-GSCs (but not PN-GSCs) were enriched for NK cell ligands and sensitive to NK-mediated cytotoxicity in vitro. While NK cells alone had no effect on HGG progression in vivo, the post-chemotherapy (TMZ) recurrence of MES-GSC-driven xenografts was aborted by timed intracranial injection of live or irradiated NK (NK92MI) cells, resulting in long term survival of animals. This curative effect declined when NK cell administration was delayed relative to TMZ exposure pointing to limits of the immune control over resurging residual tumour stem cell populations that survived chemotherapy. Overall, these results suggest that chemotherapy-dependent tumour depopulation may create a unique window of opportunity for NK-mediated intervention with curative effects restricted to a subset of HGGs driven by mesenchymal brain tumour initiating cells.
Keywords: Glioblastoma; High grade glioma; Intracranial immunotherapy; Intracranial xenografts; Mesenchymal glioma stem cells; Molecular subtypes of glioma; NK cells; Proneural glioma stem cells; Relapse; Temozolomide.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures

References
-
- Bagley SJ, Logun M, Fraietta JA, Wang X, Desai AS, Bagley LJ, Nabavizadeh A, Jarocha D, Martins R, Maloney E al (2024) Intrathecal bivalent CAR T cells targeting EGFR and IL13Rα2 in recurrent glioblastoma: phase 1 trial interim results. Nat Med 30:1320–1329. 10.1038/s41591-024-02893-z - PubMed
-
- Bosma GC, Custer RP, Bosma MJ (1983) A severe combined immunodeficiency mutation in the mouse. Nature 301:527–530. 10.1038/301527a0 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NDR Program/Montreal Children's Hospital Foundation (MCHF)
- Fellowship/Michael Whitehead and Louise Penny Endowment (MWLPE)
- Fellowship/Fonds de recherche du Quebec- Sante (FRQS)
- Fellowship/RI MUHC Desjardins and McGill Faculty of Medicine stipend
- CIHR, PJT 183971/Canadian Institutes for Health Research
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

