Rare damaging CCR2 variants are associated with lower lifetime cardiovascular risk
- PMID: 40119478
- PMCID: PMC11929344
- DOI: 10.1186/s13073-025-01456-2
Rare damaging CCR2 variants are associated with lower lifetime cardiovascular risk
Abstract
Background: Previous work has shown a role of CCL2, a key chemokine governing monocyte trafficking, in atherosclerosis. However, it remains unknown whether targeting CCR2, the cognate receptor of CCL2, provides protection against human atherosclerotic cardiovascular disease.
Methods: Computationally predicted damaging or loss-of-function (REVEL > 0.5) variants within CCR2 were detected in whole-exome-sequencing data from 454,775 UK Biobank participants and tested for association with cardiovascular endpoints in gene-burden tests. Given the key role of CCR2 in monocyte mobilization, variants associated with lower monocyte count were prioritized for experimental validation. The response to CCL2 of human cells transfected with these variants was tested in migration and cAMP assays. Validated damaging variants were tested for association with cardiovascular endpoints, atherosclerosis burden, and vascular risk factors. Significant associations were replicated in six independent datasets (n = 1,062,595).
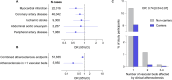
Results: Carriers of 45 predicted damaging or loss-of-function CCR2 variants (n = 787 individuals) were at lower risk of myocardial infarction and coronary artery disease. One of these variants (M249K, n = 585, 0.15% of European ancestry individuals) was associated with lower monocyte count and with both decreased downstream signaling and chemoattraction in response to CCL2. While M249K showed no association with conventional vascular risk factors, it was consistently associated with a lower risk of myocardial infarction (odds ratio [OR]: 0.66, 95% confidence interval [CI]: 0.54-0.81, p = 6.1 × 10-5) and coronary artery disease (OR: 0.74, 95%CI: 0.63-0.87, p = 2.9 × 10-4) in the UK Biobank and in six replication cohorts. In a phenome-wide association study, there was no evidence of a higher risk of infections among M249K carriers.
Conclusions: Carriers of an experimentally confirmed damaging CCR2 variant are at a lower lifetime risk of myocardial infarction and coronary artery disease without carrying a higher risk of infections. Our findings provide genetic support for the translational potential of CCR2-targeting as an atheroprotective approach.
Keywords: Atherosclerosis; CCR2; Cardiovascular disease; Genetics; Inflammation.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All studies have received ethical approvals from the respective ethical authorities and participants in all studies have provided written informed consent. Data from the UKB are available upon submission and approval of a research proposal. The UKB has institutional review board approval from the Northwest Multi‐Center Research Ethics Committee (Manchester, UK). deCODE has been approved by the National Bioethics Committee of Iceland, and the study was conducted in agreement with conditions issued by the Data Protection Authority of Iceland (VSN_14-015). All common research protocols for the TOPMed Program have been approved by the institutional review board at the University of Maryland Baltimore, whereas individual participating studies have obtained ethical approval from their local ethical authorities, as described previously [60]. The Penn Medicine BioBank is approved by the University of Pennsylvania under IRB protocol# 813913, MVP by the Veterans Affairs Central Institutional Review Board, the Geisinger DiscovEHR-MyCode cohort by Geisinger Institutional Review Boards, and MGBB by the Ethics Committee of Mass General Brigham. Consent for publication: Not applicable. Competing interests: MKG has received consulting fees from Tourmaline Bio Inc., and Gerson Lehrman Group Inc. (unrelated to this work) and is a member of the Editorial Board of Neurology. SMD receives research support from RenalytixAI and personal fees from Calico Labs, both outside the current work. MEM receives funding from Regeneron Pharmaceutical Inc. unrelated to this work. RD has received research support from AstraZeneca and Goldfinch Bio, not related to this work. BMP serves on the Steering Committee of the Yale Open Data Access Program funded by Johnson & Johnson. CDA has received sponsored research support from Bayer AG, has consulted for ApoPharma, and is a member of the Editorial Board of Neurology. LMR is a consultant for the TOPMed Administrative Coordinating Center (through WeStat). The remaining authors have no conflicts of interest to disclose.
Figures




Update of
-
Rare damaging CCR2 variants are associated with lower lifetime cardiovascular risk.medRxiv [Preprint]. 2024 Jun 26:2023.08.14.23294063. doi: 10.1101/2023.08.14.23294063. medRxiv. 2024. Update in: Genome Med. 2025 Mar 21;17(1):27. doi: 10.1186/s13073-025-01456-2. PMID: 37645892 Free PMC article. Updated. Preprint.
References
-
- Guedeney P, Claessen BE, Kalkman DN, Aquino M, Sorrentino S, Giustino G, Farhan S, Vogel B, Sartori S, Montalescot G, et al. Residual inflammatory risk in patients with low LDL cholesterol levels undergoing percutaneous coronary intervention. J Am Coll Cardiol. 2019;73:2401–9. 10.1016/j.jacc.2019.01.077. - PubMed
-
- Ridker PM. How common is residual inflammatory risk? Circ Res. 2017;120:617–9. 10.1161/CIRCRESAHA.116.310527. - PubMed
-
- Lutgens E, Atzler D, Doring Y, Duchene J, Steffens S, Weber C. Immunotherapy for cardiovascular disease. Eur Heart J. 2019;40:3937–46. 10.1093/eurheartj/ehz283. - PubMed
-
- Weber C, Noels H. Atherosclerosis: current pathogenesis and therapeutic options. Nat Med. 2011;17:1410–22. 10.1038/nm.2538. - PubMed

