Substrate flexibility of Mycoplasma fermentans mf1 phosphorylcholine transferase
- PMID: 40119989
- PMCID: PMC11982090
- DOI: 10.1007/s10719-025-10181-2
Substrate flexibility of Mycoplasma fermentans mf1 phosphorylcholine transferase
Abstract
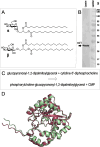
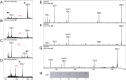
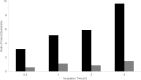
Zwitterionic modifications of glycans such as phosphorylcholine or phosphoethanolamine occur in a wide range of prokaryotic and eukaryotic organisms and are known for interaction with the mammalian immune system. Unlike the biosynthesis of membrane phospholipids which is well elucidated, very little is known about the transfer of zwitterionic phosphodiester moieties onto glycoconjugates. The presence and function of relevant enzymes has been suggested by gene knockout or mutation and corresponding aberrant phosphorylcholine metabolism. In the current study, the Mycoplasma fermentans phosphorylcholine transferase mf1, with previously confirmed in-vitro activity synthesizing phosphorylcholine-α-glucosyl-1,2-dipalmitoyl glycerol, is demonstrated to not only transfer phosphorylcholine but also phosphoethanolamine from CDP-ethanolamine. Moreover, mf1 is capable of using the β-configuration of the presumed natural substrate but transfers neither to simpler substrates with glucose moieties such as β-D-octyl-glucopyranoside nor to an extended lipid substrate with an additional galactose residue. These findings suggest a certain, but limited, substrate flexibility for bacterial PC-transferases. Mf1 activity is inhibited by β-glycerophosphate, an isomer of part of CDP-glycerol which is known to compete with CDP-ribitol in enzymatic reactions catalyzed by fukutin, a human protein sharing structural homology with mf1. For the first time, a phosphorylcholine transferase, mf1, could be biochemically characterized in vitro and its lipid products with zwitterionic phosphodiesters attached could be detected specifically with the pentraxin serum amyloid P.
Keywords: Mycoplasma fermentans; LicD; PC-transferase; Phosphocholine; Phosphorylcholine.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: I.B.H.W. is a member of the editorial board of the Glycoconjugate Journal.
Figures


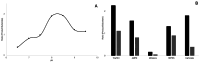
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

