Protocol for the generation and assessment of functional macrophages from mouse bone marrow cells
- PMID: 40120113
- PMCID: PMC11976235
- DOI: 10.1016/j.xpro.2025.103706
Protocol for the generation and assessment of functional macrophages from mouse bone marrow cells
Abstract
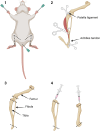
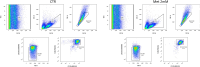
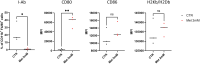
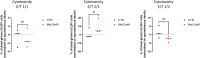
Macrophages play essential roles in tissues, wherein they exert beneficial or detrimental functions depending on the signals they encounter during their differentiation. Here, we present a protocol to differentiate mouse bone marrow cells into macrophages under specific cues to evaluate their impact on macrophage phenotypic acquisition. We provide detailed instructions for optimal cell culture conditions, quality controls, and examples of concluding immunological functional assays. This protocol is applicable in short- and long-term drug-based modulation of macrophage functions. For complete details on the use and execution of this protocol, please refer to Scafidi et al.1.
Keywords: Cell biology; Cell culture; Cell isolation; Immunology; Model organisms; Molecular biology.
Copyright © 2025 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures








References
-
- Scafidi A., Lind-Holm Mogensen F., Campus E., Pailas A., Neumann K., Legrave N., Bernardin F., Pereira S.L., Antony P.M.A., Nicot N., et al. Metformin impacts the differentiation of mouse bone marrow cells into macrophages affecting tumour immunity. Heliyon. 2024;10 doi: 10.1016/j.heliyon.2024.e37792. - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

