Modulation of lipid nanoparticle-formulated plasmid DNA drives innate immune activation promoting adaptive immunity
- PMID: 40120578
- PMCID: PMC12047470
- DOI: 10.1016/j.xcrm.2025.102035
Modulation of lipid nanoparticle-formulated plasmid DNA drives innate immune activation promoting adaptive immunity
Abstract
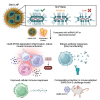
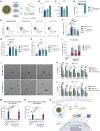
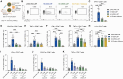
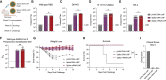
Nucleic acid vaccines have grown in importance over the past several years, with the development of new approaches remaining a focus. We describe a lipid nanoparticle-formulated DNA (DNA-LNP) formulation which induces robust innate and adaptive immunity with similar serological potency to mRNA-LNPs and adjuvanted protein. Using an influenza hemagglutinin (HA)-encoding construct, we show that priming with our HA DNA-LNP demonstrated stimulator of interferon genes (STING)-dependent upregulation and activation of migratory dendritic cell (DC) subpopulations. HA DNA-LNP induced superior antigen-specific CD8+ T cell responses relative to mRNA-LNPs or adjuvanted protein, with memory responses persisting beyond one year. In rabbits immunized with HA DNA-LNP, we observed immune responses comparable or superior to mRNA-LNPs at the same dose. In an additional model, a SARS-CoV-2 spike-encoding DNA-LNP elicited protective efficacy comparable to spike mRNA-LNPs. Our study identifies a platform-specific priming mechanism for DNA-LNPs divergent from mRNA-LNPs or adjuvanted protein, suggesting avenues for this approach in prophylactic and therapeutic vaccine development.
Keywords: DNA-LNP; T cell; adjuvanted protein; antibody; lipid nanoparticle; mRNA; plasmid DNA; vaccine.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests D.B.W. has received grant funding; participates in industry collaborations; has received speaking honoraria; and has received fees for consulting, including serving on scientific review committees. Remunerations received by D.B.W. include direct payments and equity/options. D.B.W. also discloses the following associations with commercial partners: Geneos (consultant/advisory board), AstraZeneca (advisory board, speaker), INOVIO (board of directors, consultant), Sanofi (advisory board), BBI (advisory board), Pfizer (advisory Board), and Advaccine (consultant). N.P. is named on patents describing the use of nucleoside-modified mRNA in LNPs as a vaccine platform. He has disclosed those interests fully to the University of Pennsylvania, and he has in place an approved plan for managing any potential conflicts arising from the licensing of these patents. N.P. served on the mRNA strategic advisory board of Sanofi Pasteur in 2022 and the advisory board of Pfizer in 2023 and 2024. N.P. is a member of the Scientific Advisory Board of AldexChem and BioNet-Asia. The Icahn School of Medicine at Mount Sinai has filed patent applications relating to SARS-CoV-2 serological assays, Newcastle disease virus (NDV)-based SARS-CoV-2 vaccines influenza virus vaccines, and influenza virus therapeutics, which list F.K. as a co-inventor. Mount Sinai has spun out a company, Kantaro, to market serological tests for SARS-CoV-2 and another company, CastleVax, to develop SARS-CoV-2 vaccines. F.K. is a co-founder and scientific advisory board member of CastleVax. F.K. has consulted for Merck, Curevac, GSK, Seqirus, and Pfizer and is currently consulting for 3rd Rock Ventures, Gritstone, and Avimex. The Krammer laboratory is collaborating with Dynavax on influenza vaccine development. N.J.T., D.B.W., and N.P. have filed a patent application related to aspects of this work.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

