Immunogenicity and safety of an Escherichia coli-produced bivalent human papillomavirus vaccine (Cecolin) in girls aged 9-14 years in Ghana and Bangladesh: a randomised, controlled, open-label, non-inferiority, phase 3 trial
- PMID: 40120597
- PMCID: PMC12287245
- DOI: 10.1016/S1473-3099(25)00031-3
Immunogenicity and safety of an Escherichia coli-produced bivalent human papillomavirus vaccine (Cecolin) in girls aged 9-14 years in Ghana and Bangladesh: a randomised, controlled, open-label, non-inferiority, phase 3 trial
Abstract
Background: Human papillomavirus (HPV) vaccines have been available for nearly 20 years. However, the overall coverage of girls aged 15 years and younger is low, especially in low-resource settings, where the burden of cervical cancer is highest. Increasing access and facilitating implementation of HPV vaccination will contribute to cervical cancer elimination efforts. To generate data in different dosing regimens and in low-resource settings, we aimed to evaluate the safety and immunogenicity of various schedules of an Escherichia coli-expressed bivalent HPV vaccine (2vHPV) compared with a widely used quadrivalent vaccine.
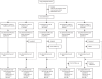
Methods: This randomised, controlled, open-label, non-inferiority, phase 3 trial enrolled healthy girls aged 9-14 years from single sites in Ghana and Bangladesh. Participants were randomly assigned via interactive web response system technology equally into five study groups, stratified by site: two doses of 2vHPV, the first at baseline and the second 6, 12, or 24 months later; a quadrivalent HPV vaccine (4vHPV) at baseline followed 24 months later by 2vHPV; or two doses of 4vHPV given 6 months apart (referent). We tested for antigen-specific (HPV-16 and HPV-18) binding antibodies by ELISA at baseline and before and 1 month after the second dose. The primary objective was to show immunological non-inferiority of the 2vHPV vaccine schedules to the referent 1 month after the second dose in the per-protocol population, with a non-inferiority margin of 0·5 for the lower bound of the 98·3% CI for the geometric mean concentration (GMC) ratio. Adverse events and serious adverse events were evaluated as secondary endpoints in the total vaccinated population. The study is registered at ClinicalTrials.gov (NCT04508309) and is completed.
Findings: Between March 15 and Nov 18, 2021, 1025 girls were enrolled and received 2vHPV at baseline and 6 months (n=205), 12 months (n=206), or 24 months (n=204); 4vHPV at baseline and 6 months (n=205); or 4vHPV at baseline and 2vHPV at 24 months (n=205). 96-99% of participants across groups were included in the per-protocol analysis. 1 month after the second dose, 2vHPV non-inferiority was shown, with GMC ratios between 1·1 and 2·4 (lower bound of the 98·3% CI of the GMC ratio between 0·9 and 1·9) for HPV-16, and between 1·3 and 1·7 (1·0 and 1·4) for HPV-18. As an exploratory objective, we assessed 2vHPV immunogenicity after one dose, finding that it was similar to that of 4vHPV up to 24 months, with GMC ratios at 24 months of 1·1 (95% CI 0·9-1·4) for HPV-16 and 1·4 (1·1-1·7) for HPV-18. The frequency of adverse events was similar across study groups, with no related unsolicited events reported. Serious adverse events were rare and none were determined to be related to vaccination.
Interpretation: Non-inferior immune responses for extended two-dose regimens of 2vHPV support dosing flexibility. For up to 24 months, one dose of 2vHPV elicited immunogenicity that was similar to one dose of 4vHPV, for which single-dose efficacy has been shown, supporting a single-dose use of 2vHPV.
Funding: The Bill & Melinda Gates Foundation and the German Federal Ministry of Education and Research and immunological testing was funded in part by the National Cancer Institute, National Institutes of Health.
Translation: For the Bengali translation of the abstract see Supplementary Materials section.
Copyright © 2025 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests We declare no competing interests.
Figures


References
-
- Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229–263. - PubMed
-
- WHO Global strategy to accelerate the elimination of cervical cancer as a public health problem. Nov 17, 2020. https://www.who.int/publications/i/item/9789240014107
-
- WHO Human papillomavirus (HPV) vaccination coverage. https://immunizationdata.who.int/global/wiise-detail-page/human-papillom...
-
- WHO Human papillomavirus vaccines: WHO position paper, December 2022. Dec 16, 2022. https://www.who.int/publications/i/item/who-wer9750-645-672
-
- WHO . 2nd edn. World Health Organization; 2024. Considerations for human papillomavirus (HPV) vaccine product choice.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

