10 degree C static storage of porcine donation after circulatory death livers improves biliary viability and mitigates ischemia-reperfusion injury
- PMID: 40120647
- PMCID: PMC12221823
- DOI: 10.1016/j.ajt.2025.03.018
10 degree C static storage of porcine donation after circulatory death livers improves biliary viability and mitigates ischemia-reperfusion injury
Abstract
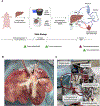
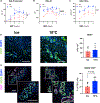
Optimized static cold storage has the potential to improve the preservation of organs most vulnerable to ischemia-reperfusion injury. Data from lung transplantation suggest that storage at 10 °C improves mitochondrial preservation and subsequent allograft function compared with conventional storage on ice. Using a porcine model of donation after circulatory death, we compared static storage of livers at 10 °C to ice. Livers (N = 5 per group) underwent 10 hours of storage followed by 4 hours of normothermic machine perfusion (NMP) for real-time allograft assessment. Allografts were compared using established NMP viability criteria, tissue immunostaining, and tissue metabolomics. Livers stored at 10 °C demonstrated lower portal venous vascular resistance and greater hepatic artery vasoresponsiveness. Lactate clearance during NMP was similar between the groups. Livers stored at 10 °C showed favorable biochemical parameters of biliary viability, including greater bile volume, pH, and bicarbonate. Metabolomics analysis revealed increased aerobic respiration, improved electron transport chain function, and less DNA damage after reperfusion of livers stored at 10 °C. Static storage of donation after circulatory death livers with extended cold ischemic time at 10 °C demonstrates superior allograft function with evidence of improved biliary viability and mitochondrial function compared with ice. These data suggest that storage at 10 °C should be considered for translation to clinical practice.
Keywords: 10 °C static storage; biliary viability; donation after circulatory death; ischemia-reperfusion injury; liver transplantation; organ preservation.
Copyright © 2025 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors of this manuscript have no conflicts of interest to disclose as described by American Journal of Transplantation.
Figures





References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

