Lung rehabilitation using xenogeneic cross-circulation does not lead to hyperacute rejection in a human lung transplantation model
- PMID: 40120998
- PMCID: PMC12191440
- DOI: 10.1016/j.healun.2025.02.1696
Lung rehabilitation using xenogeneic cross-circulation does not lead to hyperacute rejection in a human lung transplantation model
Abstract
Background: Access to life-saving lung transplantation remains limited by a shortage of donor organs. We have previously described rehabilitation of discarded human donor lungs to a quality suitable for transplantation using cross-circulation of whole blood between xeno-support swine and human lungs. However, the immunologic implications of transplanting rehabilitated lungs remain unknown.
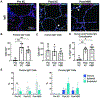
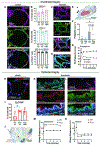
Methods: Human donor lungs declined for clinical transplantation (N = 5) and underwent xenogeneic cross-circulation (XC) for up to 12 hours. To model subsequent human transplantation, lungs were re-exposed to autologous human whole blood via normothermic ex vivo machine perfusion for up to 6 hours. Upon human blood re-exposure (HBR), lungs were evaluated for evidence of hyperacute rejection (HAR) through physiologic assessments and tissue analyses including histology, immunostaining, and flow cytometry.
Results: Upon HBR, lungs showed no significant change in physiologic function relative to the end of cross-circulation (PaO2/FiO2: p = 0.41; vascular resistance: p = 0.27; dynamic compliance: p = 0.24) and histologic features of HAR were absent in all lungs. Despite pulmonary deposition of porcine IgG during cross-circulation, HBR resulted in decreased complement deposition (p = 0.019) with no change in membrane attack complex formation (p = 0.65) or apoptotic signaling (p = 0.93). Endothelial integrity was maintained after HBR with preservation of microvascular tight junctions, decreasing endothelial injury marker p-selectin (p = 0.34), and intact vascular response to alpha-adrenergic stimulation.
Conclusions: Our findings indicate that transient exposure of human donor lungs to XC does not result in HAR upon simulated human transplantation, representing an important step toward clinical translation of this donor organ rehabilitation platform.
Keywords: human lung transplantation model; lung rehabilitation; lung transplantation; transplant immunology; xenogeneic cross-circulation.
Copyright © 2025 International Society for the Heart and Lung Transplantation. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Disclosures
MB is an inventor on a patent related to this work filed by Columbia University (No. WO2018013849A1) filed July 13, 2017 and published January 18, 2018. WKW, RU, and MB are inventors on a patent application related to this work filed by Vanderbilt University (No. 63/165,773) filed March 25, 2021. All other authors declare they have no competing interests.
Figures




References
-
- Gouchoe DA, Sanchez PG, D’Cunha J, et al. Ex vivo lung perfusion in donation after circulatory death: A post hoc analysis of the Normothermic Ex Vivo Lung Perfusion as an Assessment of Extended/Marginal Donors Lungs trial. J Thorac Cardiovasc Surg. 2024;168(3):724–734.e7. doi: 10.1016/j.jtcvs.2024.03.011 - DOI - PubMed
MeSH terms
Grants and funding
- S10 OD034315/OD/NIH HHS/United States
- S10 OD023475/OD/NIH HHS/United States
- T32 CA106183/CA/NCI NIH HHS/United States
- P30 DK058404/DK/NIDDK NIH HHS/United States
- P30 EY008126/EY/NEI NIH HHS/United States
- T32 HL160508/HL/NHLBI NIH HHS/United States
- R01 HL160551/HL/NHLBI NIH HHS/United States
- P30 CA068485/CA/NCI NIH HHS/United States
- U24 DK059637/DK/NIDDK NIH HHS/United States
- T32 GM007347/GM/NIGMS NIH HHS/United States
- P30 DK020593/DK/NIDDK NIH HHS/United States
- R01 HL171577/HL/NHLBI NIH HHS/United States
- S10 OD012324/OD/NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

