A SITC vision: adapting clinical trials to accelerate drug development in cancer immunotherapy
- PMID: 40121030
- PMCID: PMC11931932
- DOI: 10.1136/jitc-2024-010760
A SITC vision: adapting clinical trials to accelerate drug development in cancer immunotherapy
Abstract
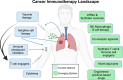
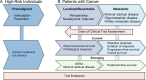
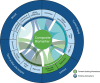
Clinical trials of cancer immunotherapy (IO) were historically based on a drug development paradigm built for chemotherapies. The remarkable clinical activity of programmed cell death protein 1/programmed death ligand 1 blockade, chimeric antigen receptor-T cells, and T cell engagers yielded new insights into how the mechanistic underpinnings of IO are reflected in the clinic. These insights and the sheer number of novel immunotherapies currently in the pipeline have made it clear that our strategies and tools for IO drug development must adapt. Recent innovations like engineered T cells and tumor-infiltrating lymphocytes demonstrate that immune-based treatments may rely on real-time manufacturing programs rather than off-the-shelf drugs. We now recognize adoptively transferred cells as living drugs. Progression criteria have been redefined due to the unique response patterns of IO. Harnessing the power of both biomarkers and the neoadjuvant setting earlier in drug development is of broad interest. The US Food and Drug Association is increasingly impacting the design of trials with respect to dose optimization and clinical endpoints. The use of novel endpoints such as pathologic complete/major response, treatment-free survival, and minimal residual disease is becoming more common. There is growing acceptance of using patient-reported outcomes as trial endpoints to better measure the true clinical benefit and impact of novel IO agents on quality of life. New opportunities created by modern data science and artificial intelligence to inform and accelerate drug development continue to emerge. The importance of streamlining the clinical research ecosystem and enhancing clinical trial access to facilitate the enrollment of diverse patient populations is broadly recognized. Patient advocacy is critical both to drive the science of IO, and to promote patient satisfaction. To capitalize on these opportunities, the Society for Immunotherapy of Cancer (SITC) has established a goal of at least 100 new, unique IO approvals over the next 10 years. Accordingly, SITC has developed initiatives designed to integrate the viewpoints of diverse stakeholders and galvanize the field in further adapting clinical trials to the unique features of IO, moving us closer to our ultimate goal of using IO to cure and prevent cancer.
Keywords: Adoptive cell therapy - ACT; Biomarker; Immune Checkpoint Inhibitor; Patient reported outcome - PRO; Tumor microenvironment - TME.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: TM—Researcher: Regeneron, Genentech, Bristol Myers Squibb, Merck, and Boehringer Ingelheim; Consultant/Advisor/Speaker: Advisory and/or Data Safety Monitoring Boards for Rockefeller University, Regeneron, AbbVie, Merck, Bristol Meyers Squibb, Boehringer Ingelheim, Atara, AstraZeneca, Genentech, Celldex, Chimeric, Dren Bio, Glenmark, Simcere, Surface, G1 Therapeutics, NGM Bio, DBV Technologies, Arcus, and Astellas. JJL—Researcher: AbbVie, Astellas, Astrazeneca, Bristol Myers Squibb, Corvus, Day One, EMD Serono, F-star, Genmab, Ikena, Immatics, Incyte, Kadmon, KAHR, Macrogenics, Merck, Moderna, Nektar, Next Cure, Numab, Palleon, Pfizer, Replimune, Rubius, Servier, Scholar Rock, Synlogic, Takeda, Trishula, Tizona, Xencor. Consultant/Advisor/Speaker: Abbvie, Agenus, Alnylam, Atomwise, Bayer, Bristol Myers Squibb, Castle, Checkmate, Codiak, Crown, Cugene, Curadev, Day One, Eisai, EMD Serono, Endeavor, Flame, G1 Therapeutics, Genentech, Gilead, Glenmark, HotSpot, Kadmon, KSQ, Janssen, Ikena, Inzen, Immatics, Immunocore, Incyte, Instil, IO Biotech, Macrogenics, Merck, Mersana, Nektar, Novartis, Partner, Pfizer, Pioneering Medicines, PsiOxus, Regeneron, Replimmune, Ribon, Roivant, Servier, Stingthera, Synlogic, Synthekine. BH—Nothing to disclose. JP—Consultant/Advisor/Speaker: Seagen, Gilead, Pfizer. CS—Nothing to disclose. VA—Nothing to disclose. AWS—Researcher: Biohaven Pharmaceuticals, Replimune, Morphogenesis, Shattuck Laboratories, Regeneron, Merck. Consultant/ Advisor/ Speaker: Instil Bio, Signatera, Merck and Regeneron; Royalty and Patent Beneficiary: Up To Date. Publicly Traded Stocks: Illumina. PJR—Employee: Novigenix, SA; Researcher: Roche, pRED,Schilieren, CH; Consultant/Advisor/ Speaker: Enterome, Transgene, Maxivax. EG-M—Nothing to disclose. LAE—Researcher: Abbvie, AstraZeneca, Bolt Therapeutics, Bristol Meyers Squibb, Compugen, Corvus, CytomX, EMD Serono, Genentech, F Hoffmann-La Roche, Immune-Onc, Merck, NextCure, Silverback, Takeda, Tempest; Consultant/Advisor/Speaker: AstraZeneca, BioLineRx, DNAMx, Genentech, F Hoffmann-La Roche, GPCR, Gilead, Immune-Onc, Immunitas, Immutep, Lilly, Macrogenics, Mersana, Shionogi; Royalty and Patent Beneficiary: potential for royalties in the future from MolecuVax; Publicly Traded Stocks: potential for stock options in the future from Ankyra Therapeutics; Other: NSABP Foundation, Translational Breast Cancer Research Consortium, Breast Cancer Research Foundation, National Cancer Institute, Department of Defense, Johns Hopkins University, University of California San Francisco, Cornell University, Dana Farber Cancer Institute, Stand Up to Cancer; These are grants from non-industry entities.
Figures
References
-
- Tawbi HA, Sullivan RJ, Feltquate D, et al. Society for Immunotherapy of Cancer (SITC) checkpoint inhibitor resistance definitions: efforts to harmonize terminology and accelerate immuno-oncology drug development. J Immunother Cancer. 2023;11:e007309. doi: 10.1136/jitc-2023-007309. - DOI - PMC - PubMed
-
- Berdeja JG, Madduri D, Usmani SZ, et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): a phase 1b/2 open-label study. The Lancet. 2021;398:314–24. doi: 10.1016/S0140-6736(21)00933-8. - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
