Bacterial membrane vesicles restore gut anaerobiosis
- PMID: 40121189
- PMCID: PMC11929906
- DOI: 10.1038/s41522-025-00676-z
Bacterial membrane vesicles restore gut anaerobiosis
Abstract
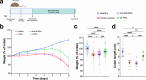
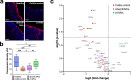
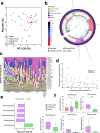
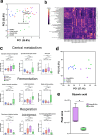
Inflammation damages the epithelial cell barrier, allowing oxygen to leak into the lumen of the gut. Respiring E. coli and other Enterobacteriaceae produce proinflammatory lipopolysaccharide, exacerbating inflammatory bowel disease. Here we show that respiring membrane vesicles (MV) from E. coli ameliorate symptoms in a mouse model of gut inflammation. Membrane vesicle treatment diminished weight loss and limited shortening of the colon. Notably, oxygenation of the colonic epithelium was significantly decreased in animals receiving wild type MVs, but not MVs from an E. coli mutant lacking cytochromes. Metatranscriptomic analysis of the microbiome shows an increase in anaerobic Lactobacillaceae and a decrease in Enterobacteriaceae, as well as a general shift towards fermentation in MV-treated mice. This is accompanied by a decrease in proinflammatory TNF-α. We report that MVs may lead to the development of a novel type of a therapeutic for dysbiosis, and for treating IBD.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: K.L. and N.P. are inventors on a patent application related to this work filed by Northeastern University: Provisional Patent Application No. 63/697942. The authors declare that they have no other competing interests.
Figures

References
-
- Roda, G. et al. Crohn’s disease. Nat. Rev. Dis. Primers6. 10.1038/s41572-020-0156-2 (2020).
-
- Kobayashi, T. et al. Ulcerative colitis. Nat. Rev. Dis. Primers6. 10.1038/s41572-020-0205-x (2020). - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

