LL37 complexed to double-stranded RNA induces RIG-I-like receptor signalling and Gasdermin E activation facilitating IL-36γ release from keratinocytes
- PMID: 40121229
- PMCID: PMC11929817
- DOI: 10.1038/s41419-025-07537-9
LL37 complexed to double-stranded RNA induces RIG-I-like receptor signalling and Gasdermin E activation facilitating IL-36γ release from keratinocytes
Abstract
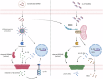
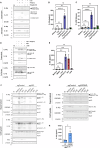
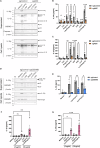
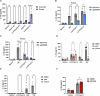
The Interleukin-36 (IL-36) cytokine family have emerged as important players in mounting an inflammatory response at epithelial barriers and tailoring appropriate adaptive immune responses. As members of the Interleukin-1 superfamily, IL-36 cytokines lack a signal peptide for conventional secretion and require extracellular proteolysis to generate bioactive cytokines. Although the IL-36 family plays an important role in the pathogenesis of plaque and pustular psoriasis, little is known about the release mechanisms of these cytokines from keratinocytes and the physiological stimuli involved. Nucleic acid released from damaged or dying keratinocytes initiates early inflammatory signals that result in the breaking of tolerance associated with psoriasis pathogenesis onset. Cathelicidin peptide, LL37 binds to DNA or double-stranded RNA (dsRNA) and activates a type I Interferon responses in plasmacytoid dendritic cells and keratinocytes. Here, we demonstrate that LL37 binds to dsRNA and induces IL-36γ release from human primary keratinocytes. LL37/dsRNA complexes activate RIG-I-like Receptor signalling, resulting in Caspase-3 and Gasdermin E (GSDME) cleavage. Subsequent GSDME pore formation facilitates IL-36γ release. This response is magnified by priming with psoriasis-associated cytokines, IL-17A and IFNγ. IL-36γ release in this manner is largely independent of cell death in primary keratinocytes and lacked extracellular proteolysis of IL-36γ. Conversely, transfection of keratinocytes directly with dsRNA synthetic analogue, Poly(I:C) induces NLRP1 inflammasome activation, which facilitates IL-36γ expression and release in a GSDMD-dependent manner. Inflammasome-associated cell death also enables extracellular processing of IL-36γ by the release of keratinocyte-derived proteases. These data highlight the distinct responses triggered by dsRNA sensors in keratinocytes. Depending on the inflammatory context and magnitude of the exogenous threat, keratinocytes will release IL-36γ coupled with cell death and extracellular cleavage or release the inactive pro-form, which requires subsequent processing by neutrophil proteases to unleash full biological activity, as occurring in psoriatic skin. Cytoplasmic sensing of dsRNA in keratinocytes mediates IL-36γ release via caspase activity and GSDM pore formation Keratinocytes release IL-36γ upon stimulation with intracellular dsRNA alone or complexed to the psoriasis-associated cathelicidin anti-microbial peptide LL37. Left: Transfected dsRNA triggers NLRP1 inflammasome assembly and IL-1β release, which can enhance IL-36γ expression, resulting in IL-36γ release and extracellular cleavage by released proteases. Right: LL37/dsRNA complexes activate a MDA5-MAVS pathway facilitating the release of IL-36γ through Caspase-3 activation and GSDME pore formation.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests. Ethics: Skin biopsies, from which primary keratinocytes were extracted, were collected with informed written consent upon approval from the Kantonale Ethikkommission Zürich (2015-0198 and 2021-00958) and all methods were conducted according to the Declaration of Helsinki Principles.
Figures


References
-
- Nishida A, Hidaka K, Kanda T, Imaeda H, Shioya M, Inatomi O, et al. Increased Expression of Interleukin-36, a Member of the Interleukin-1 Cytokine Family, in Inflammatory Bowel Disease. Inflamm Bowel Dis. 2016;22:303–14. - PubMed
-
- Russell SE, Horan RM, Stefanska AM, Carey A, Leon G, Aguilera M, et al. IL-36alpha expression is elevated in ulcerative colitis and promotes colonic inflammation. Mucosal Immunol. 2016;9:1193–204. - PubMed
-
- Frey S, Derer A, Messbacher ME, Baeten DL, Bugatti S, Montecucco C, et al. The novel cytokine interleukin-36alpha is expressed in psoriatic and rheumatoid arthritis synovium. Ann Rheum Dis. 2013;72:1569–74. - PubMed
-
- Towne JE, Sims JE. IL-36 in psoriasis. Curr Opin Pharm. 2012;12:486–90. - PubMed
MeSH terms
Substances
Grants and funding
- LF-OC-22-000968/LEO Pharma Research Foundation
- IZSEZ0_185113/Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung (Swiss National Science Foundation)
- 310030_201242/Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung (Swiss National Science Foundation)
- 4078PO_198321/Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung (Swiss National Science Foundation)
- 965135/EC | Horizon 2020 Framework Programme (EU Framework Programme for Research and Innovation H2020)
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

