Viral mediated α-synuclein overexpression results in greater transgene levels and α-synuclein overload in mice bearing kinase dead mutation of LRRK2
- PMID: 40121347
- PMCID: PMC11929741
- DOI: 10.1038/s41598-025-94165-0
Viral mediated α-synuclein overexpression results in greater transgene levels and α-synuclein overload in mice bearing kinase dead mutation of LRRK2
Abstract
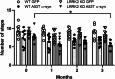
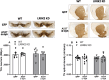
The relationship between LRRK2 mutations and susceptibility to synuclein pathology in Parkinson's disease (PD) is still unclear. We here investigate whether the mice carrying the D1994S kinase-dead (KD) mutation of LRRK2 show enhanced susceptibility to synucleinopathy. Twelve-month-old LRRK2 KD and WT mice were injected with AAV2/9 carrying human A53T α-synuclein (AAV-h-A53Tα-syn) or AAV2/9-GFP as a control. Three months after injection, α-synuclein pathology and nigrostriatal dopaminergic neuron degeneration were assessed along with motor behaviour. AAV-h-A53Tα-syn-injected LRRK2 KD mice showed a decline in stepping activity in the drag test compared to baseline levels and AAV-GFP-injected controls. This was associated with higher transgene levels and Serine129 α-syn phosphorylation in striatum and substantia nigra measured by immunohistochemistry. Total α-synuclein levels were also elevated in the substantia nigra but not striatum of AAV-h-A53Tα-syn LRRK2 KD mice compared to AAV-h-A53Tα-syn controls. Stereological counting of nigral dopaminergic neurons and densitometric analysis of striatal dopaminergic terminals did not reveal overt nigrostriatal degeneration. We conclude that silencing of kinase activity results in greater α-syn load due to greater viral transduction and/or defective α-syn clearance, possibly related to autophagy-lysosomal pathway impairment, however, with no consequence upon dopaminergic neuron survival in the mouse.
Keywords: AAV2/9; D1994S LRRK2; Kinase-dead; Parkinson’s disease; Viral vectors; Α-synuclein.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

