Truenat MTB assays for pulmonary tuberculosis and rifampicin resistance in adults and adolescents
- PMID: 40122135
- PMCID: PMC11930391
- DOI: 10.1002/14651858.CD015543.pub2
Truenat MTB assays for pulmonary tuberculosis and rifampicin resistance in adults and adolescents
Abstract
Background: Accurate and rapid diagnosis is crucial for ending the tuberculosis epidemic. Truenat assays are World Health Organization (WHO)-recommended rapid molecular diagnostic tests that detect Mycobacterium tuberculosis complex and rifampicin resistance.
Objectives: Primary objective To assess the diagnostic accuracy of Truenat assays (MTB, MTB Plus, and MTB-RIF Dx) for detecting pulmonary tuberculosis and rifampicin resistance in adults and adolescents with presumptive pulmonary tuberculosis. Secondary objectives To compare the diagnostic accuracy of Truenat assays and Xpert MTB/RIF Ultra for detecting pulmonary tuberculosis and rifampicin resistance and to investigate potential sources of heterogeneity (e.g. HIV status and smear status).
Search methods: We searched MEDLINE, Embase, Science Citation Index and Biosis previews, Global Index Medicus, SCOPUS, WHO ICTRP, and ClinicalTrials.gov for published articles and trials in progress on 16and 17 October 2023. We searched ProQuest Dissertations & Theses A&I for dissertations. We contacted tuberculosis experts for ongoing and unpublished studies. A WHO public call for data was made between 30 November 2023 and 15 February 2024.
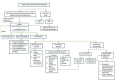
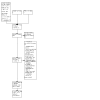
Selection criteria: We included cross-sectional and cohort studies that evaluated Truenat assays in sputum samples from adolescents and adults (aged 10 years and older). The microbiological reference standard for identifying pulmonary tuberculosis is culture. The reference standard for rifampicin resistance is a culture-based drug susceptibility test. Two review authors independently screened titles and abstracts, and assessed the full texts of potentially eligible articles. A third review author resolved any disagreements.
Data collection and analysis: We tailored and applied the QUADAS-2 and QUADAS-C tools to assess the risk of bias and applicability. Two review authors independently extracted data for each included study, and a third review author resolved any disagreements. We performed meta-analyses to estimate summary sensitivities and specificities using a bivariate model. We assessed the certainty of evidence using the GRADEpro GDT tool.
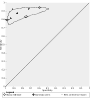
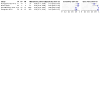
Main results: Of nine eligible articles, one contributed two distinct participant cohorts, which we considered as separate studies. Thus, we included 10 studies; three assessed Xpert Ultra. Most studies were set in low- and middle-income countries with a high tuberculosis burden. Six studies (4081 participants, 1379 with tuberculosis) assessed Truenat MTB, and four studies (3073 participants, 750 with tuberculosis) assessed Truenat MTB Plus. Two studies (966 participants, 111 with rifampicin resistance) assessed Truenat MTB-RIF Dx. Overall, the risk of bias in the included studies was low. Three of the 10 studies were judged to have high applicability concern in the patient selection domain. Detection of pulmonary tuberculosis The summary sensitivity of Truenat MTB was 87.6% (95% confidence interval (CI) 81.6 to 91.8; high-certainty evidence), and the summary specificity was 86.1% (95% CI 70.1 to 94.3; moderate-certainty evidence). For Truenat MTB Plus, the summary sensitivity was 90.6% (95% CI 83.7 to 94.8; high-certainty evidence), and the summary specificity was 95.7% (95% CI 94.7 to 96.5; high-certainty evidence). Based on the three comparative studies, the summary sensitivity of Truenat MTB was lower (81.0%, 95% CI 72.8 to 87.2) than that of Xpert Ultra (93.7%, 95% CI 90.4 to 95.9), while the summary specificity of Truenat MTB (97.0%, 95% CI 91.9 to 98.9) was marginally higher than Xpert Ultra (95.3%, 95% CI 90.9 to 97.7). Detection of rifampicin resistance The sensitivities from the two studies were 53% and 85% (moderate-certainty evidence) and specificities were both 97% (high-certainty evidence).
Authors' conclusions: Truenat MTB Plus had higher sensitivity and specificity than Truenat MTB. The high false-positive rate for Truenat MTB is a concern. The sensitivity of Xpert Ultra was significantly higher than that of Truenat MTB, while specificity was slightly lower. Evidence on the accuracy of Truenat MTB-RIF Dx was limited.
Trial registration: ClinicalTrials.gov NCT03712709 NCT02252198 NCT03303963 NCT04043390 NCT04568954 NCT05405296.
Copyright © 2025 The Authors. Cochrane Database of Systematic Reviews published by John Wiley & Sons, Ltd. on behalf of The Cochrane Collaboration.
Conflict of interest statement
Author team
LRI: none. Is employed at ICMR*.
JD: none.
MKSN: none. Is employed at ICMR*.
VAS: none. Is employed at ICMR*.
AB: none. Is employed at ICMR*.
KS: none.
PR: none. Is employed at ICMR*.
RK: none. He is on the editorial board of the Cochrane Statistical Methods group.
HDS: none. Is employed at ICMR*.
MM: none. Is employed at ICMR*.
CP: none. Is employed at ICMR*.
YT: none. She is a co‐convenor of the Cochrane Screening and Diagnostic Tests Methods Group and an editor of the Cochrane Infectious Diseases Group. She was not involved in the editorial process or decision‐making for this review.
*ICMR – this organization has published opinions in medical journals relevant to the interventions in the work and has declared its opinion on this topic.
Editors involved in editorial processing
CIDG Editor: Dr Karen Steingart reviewed data on Truenat and prepared GRADE tables for a WHO Guideline Development Meeting in December 2019 at the request of the WHO Global Tuberculosis Programme and received payment for this work. She has authored several Cochrane reviews on a similar technology: Cepheid's Xpert MTB/RIF and Xpert MTB/RIF Ultra.
DTA Editor: Dr Mariska Leeflang has no known conflicts of interest.
Figures






























Update of
- doi: 10.1002/14651858.CD015543
References
References to studies included in this review
Gomathi 2020a {published and unpublished data}
-
- Gomathi NS, Singh M, Singh UB, Myneedu VP, Chauhan DS, Sarin R, et al. Multicentric validation of indigenous molecular test Truenat™ MTB for detection of Mycobacterium tuberculosis in sputum samples from presumptive pulmonary tuberculosis patients in comparison with reference standards. Indian Journal of Medical Research 2020;152(4):378-85. [DOI: 10.4103/IJMR.IJMR_2539_19] [PMID: ] - DOI - PMC - PubMed
Gomathi 2020b {published and unpublished data}
-
- Gomathi NS, Singh M, Singh UB, Myneedu VP, Chauhan DS, Sarin R, et al. Multicentric validation of indigenous molecular test Truenat™ MTB for detection of Mycobacterium tuberculosis in sputum samples from presumptive pulmonary tuberculosis patients in comparison with reference standards. Indian Journal of Medical Research 2020;152(4):378-85. [DOI: 10.4103/IJMR.IJMR_2539_19] [PMID: ] - DOI - PMC - PubMed
Gomathi 2020c {published and unpublished data}
-
- Gomathi NS, Singh M, Myneedu VP, Chauhan DS, Tripathy SP, Sarin R, et al. Validation of an indigenous assay for rapid molecular detection of rifampicin resistance in presumptive multidrug-resistant pulmonary tuberculosis patients. Indian Journal of Medical Research 2020;152(5):482-9. [DOI: 10.4103/IJMR.IJMR_2557_19] [PMID: ] - DOI - PMC - PubMed
Jose 2024 {unpublished data only}
-
- Jose et al. Diagnostic accuracy of Truenat MTB Plus for the detection of pulmonary and extrapulmonary tuberculosis. Unpublished. - PubMed
Mangayarkarasi 2019 {published data only}
-
- Mangayarkarasi V, Sneka P, Sujith R, Jayaprakash T. Ergonomic diagnostic tool based on Chip Mini RT-PCR for diagnosis of pulmonary and extra pulmonary tuberculosis. Journal of Pure and Applied Microbiology 2019;13(2):1185-90. [DOI: 10.22207/JPAM.13.2.58] - DOI
Meena 2023 {published data only}
-
- Meena KS, Tiwari RK, Soni S, Veshar HK, Samaria A, Kant A. To evaluate the sensitivity, specificity of real time PCR (TrueNAT) assay in case detection of Mycobacterium tuberculosis in presumptive pulmonary tuberculosis cases. International Journal of Pharmaceutical and Clinical Research 2023;15(5):379-82.
Ngangue 2022 {published data only}
-
- Ngangue YR, Mbuli C, Neh A, Nshom E, Koudjou A, Palmer D, et al. Diagnostic accuracy of the Truenat MTB Plus assay and comparison with the Xpert MTB/RIF assay to detect tuberculosis among hospital outpatients in Cameroon. Journal of Clinical Microbiology 2022;60(8):e0015522. [DOI: 10.1128/JCM.00155-22] [PMID: ] - DOI - PMC - PubMed
Penn‐Nicholson 2021 {published and unpublished data}
Ssengooba 2024 {unpublished data only}
-
- Ssengooba W, Katamba A, Sserubiri J, Semugenze D, Nyombi A, Byaruhanga R, et al. Performance evaluation of Truenat MTB and Truenat MTB-RIF DX assays in comparison to gene XPERT MTB/RIF ultra for the diagnosis of pulmonary tuberculosis in Uganda. BMC infectious diseases 2024;24(1):190. [DOI: 10.1186/s12879-024-09063-z] [PMID: ] - DOI - PMC - PubMed
Theron 2024 {unpublished data only}
-
- Theron et al. Evaluation of Truenat (including Ultima) as a low-complexity molecular nucleic acid amplification test (mNAAT) for pulmonary TB diagnosis in comparison with Xpert Ultra and culture in sputum from people in South Africa. Unpublished.
References to studies excluded from this review
Akhtar 2022 {published data only}
-
- Akhtar S, Kaur A, Kumar D, Sahni B, Chouhan R, Tabassum N, et al. Diagnostic accuracy between CBNAAT, TrueNat, and smear microscopy for diagnosis of pulmonary tuberculosis in Doda district of Jammu and Kashmir – a comparative study. Journal of Clinical and Diagnostic Research 2022;16(11):DC08-12. [DOI: 10.7860/jcdr/2022/59404.17055] - DOI
Badola 2023 {published data only}
Dahiya 2023 {published data only}
Georghiou 2021 {published data only}
-
- Georghiou SB, Gomathi NS, Rajendran P, Nagalakshmi V, Prabakaran L, Prem Kumar MM, et al. Accuracy of the Truenat MTB-RIF Dx assay for detection of rifampicin resistance-associated mutations. Tuberculosis (Edinburgh, Scotland) 2021;127:102064. [DOI: 10.1016/j.tube.2021.102064] [PMID: ] - DOI - PubMed
Inamdar 2021 {published data only}
-
- Inamdar A. P6-34: prevalence of rifampicin resistance in patients with pulmonary tuberculosis in western India using automated TrueNat assay. Respirology (Carlton, Vic.) 2021;26(S3):240. [DOI: 10.1111/resp.14150_400] - DOI
Jose 2021 {published data only}
-
- Jose RA, Gopal K, Johnson AK, Samuel JA, Abraham SS, Goswami T, et al. Evaluation of truenat MTB/RIF test in comparison with microscopy and culture for diagnosis of extrapulmonary tuberculosis in a tertiary care centre. Journal of Clinical and Diagnostic Research 2021;15(1):DC05-09. [DOI: 10.7860/jcdr/2021/46815.14432] - DOI
Kambli 2020 {published data only}
-
- Kambli P, Rodrigues C. Molecular diagnosis of drug resistance in Mycobacterium tuberculosis. Advances in Molecular Pathology 2020;3(1):87-95. [DOI: 10.1016/j.yamp.2020.07.008] - DOI
Kumara 2021 {published data only}
-
- Kumara P, Kumari P, Mishra B. Truenat MTB Plus: how truly can it diagnose EPTB? Indian Journal of Medical Microbiology 2021;39(Suppl):S90. [DOI: 10.1016/j.ijmmb.2021.08.313] - DOI
MacLean 2022 {published data only}
-
- MacLean EL. Expanding the Use Cases of Molecular Testing for Tuberculosis [Thesis]. Montreal (Canada): McGill University, 2022.
Meaza 2021 {published data only}
NCT03712709 {published data only}
-
- NCT03712709. Clinical evaluation of the Truenat point-of-care tuberculosis diagnostic test [Prospective, multicentre trial to assess the diagnostic accuracy of the Truenat assays at intended settings of use]. clinicaltrials.gov/study/NCT03712709 (first received 19 August 2018). [CLINICALTRIALS.GOV: NCT03712709]
Nikam 2013 {published and unpublished data}
Nikam 2014 {published and unpublished data}
-
- Nikam C, Kazi M, Nair C, Jaggannath M, Manoj MM, Vinaya RV, et al. Evaluation of the Indian Truenat micro RT-PCR device with GeneXpert for case detection of pulmonary tuberculosis. International Journal of Mycobacteriology 2014;3(3):205-10. [DOI: 10.1016/J.IJMYCO.2014.04.003] [PMID: ] - DOI - PubMed
Sharma 2021 {published data only}
Sharma 2022 {published data only}
Sharma 2023 {published data only}
-
- Sharma K, Sharma M, Gupta N, Modi T, Joshi H, Shree R, et al. Determining the diagnostic potential of Truenat MTB Plus for Tubercular lymphadenitis and detection of drug resistance and a comparison with GeneXpert Ultra. Tuberculosis (Edinburgh, Scotland) 2023;142:102379. [DOI: 10.1016/j.tube.2023.102379] [PMID: ] - DOI - PubMed
Sharma 2024a {published data only}
Sharma 2024b {published data only}
Shireesha 2020 {published data only}
-
- Shireesha D, Surekha A, Renuka Devi A, Nagajyothi B, Vijayalakshmi J. Early diagnosis and drug resistance detection of pulmonary tuberculosis by TRUENAT and LPA in a tertiary care hospital. BMC Infectious Diseases 2020;20(S1):2-3. [DOI: 10.1186/s12879-020-05038-y] - DOI
Singh 2020 {published data only}
-
- Singh DP, Ghosh SK. Molecular assays as initial tests for the diagnosis of tuberculosis. Journal of the Indian Medical Association 2020;118(2):31-3. [WEBPAGE: https://www.onlinejima.com/read_journals.php?article=355]
Singh 2023 {published data only}
Vajravelu 2022 {published data only}
-
- Vajravelu LK, Thulukanam J, Venkatesan B, Ravi S, Muthamilan OL. Episode of endometrial tuberculosis among infertile patients In 2019–2021. Journal of Pharmaceutical Negative Results 2022;13(5):2005-8. [DOI: 10.47750/pnr.2022.13.%20S05.318] - DOI
Valsan 2022 {published data only}
-
- Valsan PM, Sudarasana J. Comparison of TrueNat polymerase chain reaction and mycobacterium growth indicator tube culture in the diagnosis of pulmonary and extrapulmonary tuberculosis. Journal of The Academy of Clinical Microbiologists 2022;24(1):21-5. [DOI: 10.4103/jacm.jacm_6_22] - DOI
Vijayalakshmi 2019 {published data only}
-
- Vijayalakshmi J, Surekha A, Renuka Devi A, Uma Devi S. Truenat – a novel diagnostic tool for rapid detection of mycobacterium tuberculosis and rifampicin resistance in pulmonary samples. International Journal of Current Microbiology and Applied Sciences 2019;8(10):1260-7. [DOI: 10.20546/ijcmas.2019.810.148] - DOI
References to ongoing studies
NCT02252198 {unpublished data only}
-
- NCT02252198. Evaluation of non-inferiority of two fast follower nucleic acid amplification tests (FIND) [Evaluation of non-inferiority of two fast follower nucleic acid amplification tests for the diagnosis of pulmonary tuberculosis in comparison to Geneexpert MTB/RIF]. clinicaltrials.gov/show/NCT02252198 (first received 30 September 2014). [CLINICALTRIALS.GOV: NCT02252198]
NCT03303963 {unpublished data only}
-
- NCT03303963. DIAgnostics for Multidrug resistant tuberculosis in Africa (DIAMA) [Culture free diagnosis and follow-up of multidrug resistant tuberculosis patients]. clinicaltrials.gov/show/NCT03303963 (first received 6 October 2017). [CLINICALTRIALS.GOV: NCT03303963]
NCT04043390 {unpublished data only}
-
- NCT04043390. A one-stop shop for the same day diagnosis and management of TB and HIV. clinicaltrials.gov/show/NCT04043390 (first received 2 August 2019). [CLINICALTRIALS.GOV: NCT04043390]
NCT04568954 {unpublished data only}
-
- NCT04568954. TB-CAPT CORE Truenat trial [Molbio Truenat TB platform combined with the Truenat TB assays for detection of tuberculosis and rifampicin resistance in adults with presumptive pulmonary tuberculosis at primary-level diagnostic centres in Tanzania and Mozambique: a pragmatic, cluster-randomized controlled trial]. clinicaltrials.gov/show/NCT04568954 (first received 29 September 2020). [CLINICALTRIALS.GOV: NCT04568954]
NCT05405296 {unpublished data only}
-
- NCT05405296. Evaluation of the Truenat™MTB Plus/COVID-19 test for TB (tuberculosis) and COVID-19 (SARS-CoV2) (Truenat COMBO) [Performance evaluation of the Molbio Diagnostics Truenat™MTB Plus/COVID-19 for TB and COVID-19 case detection using prospectively collected NP (nasopharyngeal) swabs and sputum samples from participants with symptoms suggestive of TB]. clinicaltrials.gov/show/NCT05405296 (first received 6 June 2022). [CLINICALTRIALS.GOV: NCT05405296]
Additional references
Acharya 2020
Arora 2020
Balshem 2011
Beall 2019
Blakemore 2010
Boehme 2007
-
- Boehme CC, Nabeta P, Henostroza G, Raqib R, Rahim Z, Gerhardt M, et al. Operational feasibility of using loop-mediated isothermal amplification for diagnosis of pulmonary tuberculosis in microscopy centers of developing countries. Journal of Clinical Microbiology 2007;45(6):1936-40. [DOI: 10.1128/JCM.02352-06] [PMID: ] - DOI - PMC - PubMed
Cepheid 2022a
-
- Cepheid International. Xpert® MTB/RIF. https://www.cepheid.com/en-US/tests/tb-emerging-infectious-diseases/xper... (accessed 20 October 2022).
Cepheid 2022b
-
- Cepheid International. Xpert ® MTB/RIF Ultra. https://www.cepheid.com/en-GB/tests/tb-emerging-infectious-diseases/xper... (accessed 20 October 2022).
Chakravorty 2017
-
- Chakravorty S, Simmons AM, Rowneki M, Parmar H, Cao Y, Ryan J, et al. The new Xpert MTB/RIF Ultra: improving detection of Mycobacterium tuberculosis and resistance to rifampin in an assay suitable for point-of-care testing. MBio 2017;8(4):e00812-17. [DOI: 10.1128/MBIO.00812-17] [PMID: ] - DOI - PMC - PubMed
Crudu 2012
-
- Crudu V, Stratan E, Romancenco E, Allerheiligen V, Hillemann A, Moraru N. First evaluation of an improved assay for molecular genetic detection of tuberculosis as well as rifampin and isoniazid resistances. Journal of Clinical Microbiology 2012;50(4):1264-9. [DOI: 10.1128/JCM.05903-11] [PMID: ] - DOI - PMC - PubMed
GRADEpro GDT [Computer program]
-
- GRADEpro GDT. Version accessed 15 January 2023. Hamilton (ON): McMaster University (developed by Evidence Prime), 2023. Available at https://www.gradepro.org.
Hain Lifescience 2022
-
- Hain Lifescience. GenoType MTBDRplus VER 2.0 – Your Test System for a Fast and Reliable Way to detect MDR-TB [Identification of the M. tuberculosis complex and its resistance to Rifampicin and/or Isoniazid from pulmonary clinical specimens or cultivated samples]. Available at https://www.hain-lifescience.de/en/products/microbiology/mycobacteria/tu... (accessed 6 December 2022).
Heemskerk 2015
-
- Heemskerk D, Caws M, Marais B, Farrar J. Chapter 3: clinical manifestations. In: Tuberculosis in Adults and Children. London (UK): SpringerBriefs in Public Health, 2015:17-26. [DOI: 10.1007/978-3-319-19132-4] [978-3-319-19132-4] - DOI
Hooja 2011
-
- Hooja S, Pal N, Malhotra B, Goyal S, Kumar V, Vyas L. Comparison of Ziehl Neelsen & Auramine O staining methods on direct and concentrated smears in clinical specimens. Indian Journal of Tuberculosis 2011;58(2):72-6. [PMID: ] - PubMed
Jang 2020
Kay 2020
-
- Kay AW, González Fernández L, Takwoingi Y, Eisenhut M, Detjen AK, Steingart KR, et al. Xpert MTB/RIF and Xpert MTB/RIF Ultra assays for active tuberculosis and rifampicin resistance in children. Cochrane Database of Systematic Reviews 2020, Issue 8. Art. No: CD013359. [COCHRANE: CD013359] [DOI: 10.1002/14651858.CD013359.pub2] [PMID: ] - DOI - PMC - PubMed
Kay 2022
-
- Kay AW, Ness T, Verkuijl SE, Viney K, Brands A, Masini T, et al. Xpert MTB/RIF Ultra assay for tuberculosis disease and rifampicin resistance in children. Cochrane Database of Systematic Reviews 2022, Issue 9. Art. No: CD013359. [COCHRANE: CD013359] [DOI: 10.1002/14651858.CD013359.pub3] [PMID: ] - DOI - PMC - PubMed
Kik 2014
Kumari 2020
-
- Kumari P, Thakur JK, Kumar P, Kumar R, Parekh D. Comparison of LJ medium and BACTEC MGIT 960 culture system for the diagnosis of tuberculosis. Journal of Clinical and Diagnostic Research 2020;14(12):DC9-13. [DOI: 10.7860/JCDR/2020/46890.14304] - DOI
Lee 2019
-
- Lee DJ, Kumarasamy N, Resch SC, Sivaramakrishnan GN, Mayer KH, Tripathy S, et al. Rapid, point-of-care diagnosis of tuberculosis with novel Truenat assay: cost-effectiveness analysis for India's public sector. PLOS One 2019;14(7):e0218890. [DOI: 10.1371/journal.pone.0218890] [PMID: ] - DOI - PMC - PubMed
Lewinsohn 2017
-
- Lewinsohn DM, Leonard MK, LoBue PA, Cohn DL, Daley CL, Desmond E, et al. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention clinical practice guidelines: diagnosis of tuberculosis in adults and children. Clinical Infectious Diseases 2017;64(2):111-5. [DOI: 10.1093/CID/CIW778] [PMID: ] - DOI - PMC - PubMed
Molbio 2019
-
- Molbio Diagnostics Private Limited. Truenat® MTB: Chip-based Real Time PCR Test for Mycobacterium tuberculosis. https://www.molbiodiagnostics.com/product_details.php?id=1 (accessed 21 October 2022).
Molbio 2020
-
- Molbio Diagnostics Private Limited. Truenat® MTB Plus: Chip-based Real Time PCR Test for Mycobacterium tuberculosis. https://www.molbiodiagnostics.com/product_details.php?id=49 (accessed 21 October 2022).
Nathavitharana 2017
-
- Nathavitharana RR, Cudahy PG, Schumacher SG, Steingart KR, Pai M, Denkinger CM. Accuracy of line probe assays for the diagnosis of pulmonary and multidrug-resistant tuberculosis: a systematic review and meta-analysis. European Respiratory Journal 2017;49(1):1601075. [DOI: 10.1183/13993003.01075-2016] [PMID: ] - DOI - PMC - PubMed
Pandey 2008
-
- Pandey BD, Poudel A, Yoda T, Tamaru A, Oda N, Fukushima Y, et al. Development of an in-house loop-mediated isothermal amplification (LAMP) assay for detection of Mycobacterium tuberculosis and evaluation in sputum samples of Nepalese patients. Journal of Medical Microbiology 2008;57(Pt 4):439-43. [DOI: 10.1099/jmm.0.47499-0] [PMID: ] - DOI - PubMed
Pillay 2022
-
- Pillay S, Steingart KR, Davies GR, Chaplin M, De Vos M, Schumacher SG, et al. Xpert MTB/XDR for detection of pulmonary tuberculosis and resistance to isoniazid, fluoroquinolones, ethionamide, and amikacin. Cochrane Database of Systematic Reviews 2022, Issue 5. Art. No: CD014841. [DOI: 10.1002/14651858.CD014841.pub2] [PMID: ] - DOI - PMC - PubMed
Rajendran 2022
-
- Rajendran P, Kumar MP, Thiruvengadam K, Sreenivasan P, Veeraraghavan T, Ramalingam R, et al. Characterization of probes associated with rifampicin resistance in M. tuberculosis detected by GenXpert from a national reference laboratory at Chennai. Tuberculosis (Edinburgh, Scotland) 2022;133:102182. [DOI: 10.1016/J.TUBE.2022.102182] [PMID: ] - DOI - PubMed
RevMan 2024 [Computer program]
-
- Review Manager (RevMan). Version 7.12.0. The Cochrane Collaboration, 2024. Available at https://revman.cochrane.org.
Schünemann 2008
-
- Schünemann HJ, Oxman AD, Brozek J, Glasziou P, Jaeschke R, Vist GE, et al. Grading quality of evidence and strength of recommendations for diagnostic tests and strategies. BMJ (Clinical Research Ed.) 2008;336(7653):1106-10. [DOI: 10.1136/BMJ.39500.677199.AE] [PMID: ] - DOI - PMC - PubMed
Schünemann 2016
-
- Schünemann HJ, Mustafa R, Brozek J, Santesso N, Alonso-Coello P, Guyatt G, et al. GRADE Guidelines: 16. GRADE evidence to decision frameworks for tests in clinical practice and public health. Journal of Clinical Epidemiology 2016;76:89-98. [DOI: 10.1016/J.JCLINEPI.2016.01.032] [PMID: ] - DOI - PubMed
Schünemann 2020a
-
- Schünemann HJ, Mustafa RA, Brozek J, Steingart KR, Leeflang M, Murad MH, et al. GRADE guidelines: 21 part 1. Study design, risk of bias, and indirectness in rating the certainty across a body of evidence for test accuracy. Journal of Clinical Epidemiology 2020;122:129-41. [DOI: 10.1016/J.JCLINEPI.2019.12.020] [PMID: ] - DOI - PubMed
Schünemann 2020b
-
- Schünemann HJ, Mustafa RA, Brozek J, Steingart KR, Leeflang M, Murad MH, et al. GRADE guidelines: 21 part 2. Test accuracy: inconsistency, imprecision, publication bias, and other domains for rating the certainty of evidence and presenting it in evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2020;122:142-52. [DOI: 10.1016/J.JCLINEPI.2019.12.021] [PMID: ] - DOI - PubMed
Sharma 2017
-
- Sharma A, Hill A, Kurbatova E, Walt M, Kvasnovsky C, Tupasi TE, et al. Estimating the future burden of multidrug-resistant and extensively drug-resistant tuberculosis in India, the Philippines, Russia, and South Africa: a mathematical modelling study. Lancet Infectious Diseases 2017;17(7):707-15. [DOI: 10.1016/S1473-3099(17)30247-5] [PMID: ] - DOI - PMC - PubMed
Soeroto 2021
Steingart 2006
Takwoingi 2017
Takwoingi 2023
-
- Takwoingi Y, Dendukuri N, Schiller I, Rücker G, Jones HE, Partlett C, et al. Chapter 9: Understanding meta-analysis. In: Deeks JJ, Bossuyt PM, Leeflang MM, Takwoingi Y, editor(s). Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy Version 2.0 (updated July 2023). Cochrane 2023. Available from https://training.cochrane.org/handbook-diagnostic-test-accuracy/current.
Theron 2014
-
- Theron G, Zijenah L, Chanda D, Clowes P, Rachow A, Lesosky M, et al. Feasibility, accuracy, and clinical effect of point-of-care Xpert MTB/RIF testing for tuberculosis in primary-care settings in Africa: a multicentre, randomised, controlled trial. Lancet 2014;383(9915):424-35. [DOI: 10.1016/S0140-6736(13)62073-5] [PMID: ] - DOI - PubMed
UN 2015
-
- United Nations. Sustainable development goals 2015. Goal 3: Ensure healthy lives and promote well-being for all at all ages. https://www.un.org/sustainabledevelopment/health/ (accessed 16 July 2024).
Whiting 2011
WHO 2007
-
- World Health Organization. Use of liquid TB culture and drug susceptibility testing (DST) in low and medium income settings. Summary report of the Expert Group Meeting on the use of liquid media culture, Geneva, 26 March 2007. Available at: https://stoptb.org/wg/gli/assets/documents/EGM%20report_Use%20of%20Liqui....
WHO 2013
-
- World Health Organization. Automated real-time nucleic acid amplification technology for rapid and simultaneous detection of tuberculosis and rifampicin resistance: Xpert MTB/RIF assay for the diagnosis of pulmonary and extrapulmonary TB in adults and children: policy update. Available at: https://iris.who.int/handle/10665/112472. [ISBN: 9789241506335] - PubMed
WHO 2016a
-
- World Health Organization. The use of loop-mediated isothermal amplification (TB-LAMP) for the diagnosis of pulmonary tuberculosis: policy guidance. Available at: https://iris.who.int/handle/10665/249154. [ISBN: 9789241511186] [PMID: ] - PubMed
WHO 2016b
-
- World Health Organization. The use of molecular line probe assay for the detection of resistance to isoniazid and rifampicin. Policy update. 2016. Available at: https://www.who.int/publications/i/item/9789241511261. [ISBN: 9789241511261]
WHO 2017
-
- World Health Organization. WHO meeting report of a technical expert consultation: non-inferiority analysis of Xpert MTB/RIF ultra compared to Xpert MTB/RIF. Meeting report 24 March 2017. Available at: https://www.who.int/publications/i/item/WHO-HTM-TB-2017.04. [WHO REFERENCE NUMBER: WHO-HTM-TB-2017.04]
WHO 2021a
-
- World Health Organization. WHO operational handbook on tuberculosis. Module 3: diagnosis – rapid diagnostics for tuberculosis detention, 2021 update. Available at: https://www.who.int/publications/i/item/9789240030589. [ISBN: 978-92-4-003058-9]
WHO 2021b
-
- World Health Organization. Technical report on critical concentrations for drug susceptibility testing of isoniazid and the rifamycins (rifampicin, rifabutin and rifapentine). Available at: https://www.who.int/publications/i/item/9789240017283. [ISBN: 978-92-4-001728-3]
WHO 2022a
-
- World Health Organization. WHO consolidated guidelines on tuberculosis. Module 4: treatment: drug-resistant tuberculosis treatment, 2022 update. Available at: https://www.who.int/publications/i/item/9789240063129. [ISBN: 978-92-4-006312-9] - PubMed
WHO 2022b
-
- World Health Organization. WHO operational handbook on tuberculosis. Module 4: treatment – drug-susceptible tuberculosis treatment. 2022. Available at: https://www.who.int/publications/i/item/9789240050761. [ISBN: 978-92-4-005076-1] - PubMed
WHO 2024
-
- World Health Organization. WHO consolidated guidelines on tuberculosis. Module 3: diagnosis – rapid diagnostics for tuberculosis detection, third edition. Geneva: World Health Organization; 2024. Available at: www.who.int/publications/i/item/9789240089488. [ISBN: 978-92-4-008948-8]
WHO Global TB Report 2022
-
- World Health Organization. Global tuberculosis Report 2022. Available at: who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculos.... [ISBN: 978-92-4-006172-9]
WHO Global TB Report 2023
-
- World Health Organization. Global Tuberculosis Report 2023. Available at: who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculos....
WHO Global TB Report 2024
-
- World Health Organization. Global Tuberculosis Report 2024. Available at: who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculos....
World Bank 2022
-
- World Bank. Country classification – World bank country and lending groups. https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-b... (accessed 28 October 2022).
Xi 2022
Yang 2021
Zaw 2018
Zifodya 2021
-
- Zifodya JS, Kreniske JS, Schiller I, Kohli M, Dendukuri N, Schumacher SG, et al. Xpert Ultra versus Xpert MTB/RIF for pulmonary tuberculosis and rifampicin resistance in adults with presumptive pulmonary tuberculosis. Cochrane Database of Systematic Reviews 2021, Issue 5. Art. No: CD009593. [DOI: 10.1002/14651858.CD009593.pub5] [PMID: ] - DOI - PMC - PubMed
References to other published versions of this review
Inbaraj 2023
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

