Life-supporting functional kidney replacement by integration of embryonic metanephros-bladder composite tissue transplants
- PMID: 40122339
- PMCID: PMC12521919
- DOI: 10.1016/j.kint.2025.02.024
Life-supporting functional kidney replacement by integration of embryonic metanephros-bladder composite tissue transplants
Abstract
Novel transplantable organs need to be developed to address the global organ shortage. Transplantation of embryonic kidney tissue, or metanephros, facilitates glomerular and tubular maturation and offers partial organ functional support. However, adult environments do not permit exponential growth in size, limiting the life-supporting functionality and organ replacement effect of this approach. Here, we developed a novel strategy that combines the fusion of embryonic bladders with multiple anastomoses to the host ureter, enabling a significant increase in metanephros transplantation and urinary tract integration. By surgically anastomosing divided bladder segments, we reconstructed the excretory pathways by merging four metanephroi into each bladder and integrating them with the host ureter. Following the transplantation and integration of 20 metanephroi at the para-aortic region, anephric rats survived for over a month and generated approximately 50,000 nephrons in vivo. Ultrastructural and single-cell-transcriptomic analyses revealed that the maturity of the transplanted metanephroi was comparable to that of adult kidneys, although their small size likely contributed to their decreased urine concentration ability. Postoperative support helped normalize physiological homeostasis, including solute clearance, acid-base balance, electrolyte levels, and kidney hormone levels, within vital ranges. Our findings underscore the functional maturation capacity and dose-dependent therapeutic efficacy of embryonic kidney tissue, suggesting its potential as a transplantable organ system.
Keywords: embryonic kidney; homeostasis; life-support; maturation; metanephros; transplantation.
Copyright © 2025 International Society of Nephrology. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
DISCLOSURE
A patent application for the technology involved in creating an integrated MNB graft was filed on January 20, 2023 (Japanese Patent Application Number 2023–007159): the applicant was Bios Co., Ltd.; the inventors were YK, TY, and EK. TY and EK are members of Bios Co., Ltd. All the other authors declared no competing interests.
Figures
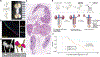





References
-
- Takebe T, Sekine K, Enomura M, et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature. 2013;499:481–484. - PubMed
-
- Sugimoto S, Kobayashi E, Fujii M, et al. An organoid-based organ-repurposing approach to treat short bowel syndrome. Nature. 2021;592:99–104. - PubMed
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

