HiSNAP trial-a multicentre, randomised, open-label, blinded end point, safety and efficacy trial of conventional (300 mg/kg) versus higher doses of acetylcysteine (450 mg/kg and 600 mg/kg) in patients with paracetamol overdose in the UK: study protocol
- PMID: 40122550
- PMCID: PMC11977480
- DOI: 10.1136/bmjopen-2024-097432
HiSNAP trial-a multicentre, randomised, open-label, blinded end point, safety and efficacy trial of conventional (300 mg/kg) versus higher doses of acetylcysteine (450 mg/kg and 600 mg/kg) in patients with paracetamol overdose in the UK: study protocol
Abstract
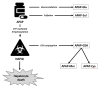
Introduction: In overdose, a larger proportion of paracetamol (acetaminophen) is converted in the liver to the toxic metabolite N-acetyl-p-benzoquinone imine (NAPQI). Glutathione (GSH) is the endogenous antioxidant that protects cells from NAPQI-induced injury. In overdose, GSH stores may become depleted, leaving NAPQI free to produce liver damage. N-Acetylcysteine (NAC) helps prevent paracetamol toxicity by replenishing liver GSH. This protective effect of NAC produces specific metabolites in the circulation. Currently, regardless of the paracetamol dose ingested, patients in the UK receive a dose of NAC based only on their weight. Basic pharmacology, mathematical modelling and observational studies suggest that this dose may be insufficient in some patients (particularly those taking a large overdose).
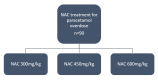
Methods and analysis: A multicentre trial, taking place across several hospitals in Scotland, UK, within Emergency Departments and Acute Medical Units. Recruitment commenced on 19 February 2024 and is anticipated to run for approximately 2 years. This is a three-group dose-finding trial, in which participants are assigned in a 1:1:1 ratio to either Standard NAC (300 mg/kg) or higher doses of 450 mg/kg (Group 1) and 600 mg/kg (Group 2). The primary outcome is the proportion of paracetamol metabolites in the circulation that are directly produced by GSH/NAC detoxification of NAPQI. A higher proportion of these metabolites will indicate that the additional NAC is reducing the amount of toxic paracetamol metabolites in the body. The study will first test the primary outcome on the HiSNAP Group 2 against Standard NAC; only if that is significant will HiSNAP Group 1 be tested against Standard NAC.
Ethics and dissemination: The HiSNAP trial has been approved by the East Midlands (Derby) Research Ethics Committee (reference 23/EM/0129), NHS Lothian Research and Development Department, and the MHRA. Results will be disseminated by peer-reviewed publication, conferences and linked on isrctn.com.
Trial registration number: ISRCTN17516192.
Keywords: CLINICAL PHARMACOLOGY; Clinical Trial; Hepatology.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY. Published by BMJ Group.
Conflict of interest statement
Competing interests: All of the HiSNAP Trial Investigators acknowledge that the work for the HiSNAP Trial is supported by Chief Scientist Office funding (TCS/21/04). JWD: MRC funding (and patent filed) for an in vitro diagnostic which could be a companion diagnostic, MRC funding for another clinical trial for the treatment of paracetamol overdose and scientific advisory board member for EU-funded TransBioLine consortium. MG: expert advisor to BMJ Best Practice (honorarium) and member of the RCEM Toxicology advisory group. CH: grants from the Centre for Precision Cell Therapy for the Liver, honoraria from Elsevier, support for meetings and travel from Royal College of Emergency Medicine (RCEM), member of the RCEM Toxicology advisory group and editor for a BMJ Group medical journal. SI: grant from the Sir Jules Thorn Charitable Trust. SI and KO: grants from the Jon Moulton Trust/National Institute for Health Research/British Heart Foundation/University of Edinburgh. KO: support for attending meetings from the Universities of Edinburgh and Nottingham, membership of UK Trial Managers Network Executive Committee and Membership of University of Edinburgh Clinical Trials MSc steering committee. CJW: recipient of the Chief Scientist Office and Medical Research Council grant funding to his institution. All other authors have no competing interest to declare.
Figures

References
-
- Humphries C, Eddleston M, Dear J. The emergency treatment of poisoning. Medicine (Abingdon) 2024;52:56–61. doi: 10.1016/j.mpmed.2023.10.004. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
