Effects of 1-year exercise in patients with atrial fibrillation: study protocol for the Norwegian Exercise in Atrial Fibrillation (NEXAF) randomised controlled trial
- PMID: 40122568
- PMCID: PMC11962797
- DOI: 10.1136/openhrt-2024-003077
Effects of 1-year exercise in patients with atrial fibrillation: study protocol for the Norwegian Exercise in Atrial Fibrillation (NEXAF) randomised controlled trial
Abstract
Introduction: Atrial fibrillation is the most prevalent sustained arrhythmia worldwide and is expected to increase substantially within the coming years. Although lifestyle changes and risk factor modification are now acknowledged as central components of atrial fibrillation management, the effects of exercise on disease-specific outcomes are still not extensively documented due to few high-quality randomised trials. The primary objective of the Norwegian Exercise in Atrial Fibrillation Trial (NEXAF) is to assess the effects of exercise over 12 months on key clinical and patient-reported outcomes in previously inactive patients with atrial fibrillation.
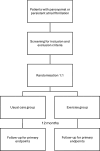
Methods and analysis: NEXAF is a multicentre, two-arm, randomised controlled trial inviting patients 18-80 years with a confirmed diagnosis of paroxysmal or persistent atrial fibrillation. Eligible patients are randomised 1:1 to either a combined supervised and eHealth-based exercise intervention or usual care for 12 months. The primary outcomes are total time in atrial fibrillation measured by insertable cardiac monitors, and disease-specific quality of life measured by the Atrial Fibrillation Effect on Quality-of-Life questionnaire.
Ethics and dissemination: Ethical approval was obtained from the Regional Ethics Committee in Mid-Norway in April 2021 (ID 213848).
Trial registration number: NCT05164718.
Keywords: Atrial Fibrillation; Cardiac Rehabilitation; Outcome Assessment, Health Care.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY. Published by BMJ Group.
Conflict of interest statement
Competing interests: M-LL has received lecture fees from Bayer, Sanofi and BMS/Pfizer. MM has received lecture fees from Astra Zeneca, Bayer, Boehringer-Ingelheim, Bristol Myers Squibb, MSD and Pfizer. HD reports institutional partnership with GE Healthcare through Centre for Innovative Ultrasound Solutions funded by the Research Council of Norway and lecture fees from Bayer AG, Boehringer Ingelheim, Pfizer, Amgen, Astra Zeneca and GE HealthCare. The remaining authors have nothing to declare.
Figures
References
-
- Visseren FLJ, et al. ESC Guidelines on cardiovascular disease prevention in clinical practice: Developed by the Task Force for cardiovascular disease prevention in clinical practice with representatives of the European Society of Cardiology and 12 medical societies With the special contribution of the European Association of Preventive Cardiology (EAPC) Eur Heart J. 2021;42:3227–337. doi: 10.1093/eurheartj/ehab484. - DOI - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous