Use of inhaled corticosteroids in bronchiectasis: data from the European Bronchiectasis Registry (EMBARC)
- PMID: 40122611
- PMCID: PMC12128780
- DOI: 10.1136/thorax-2024-221825
Use of inhaled corticosteroids in bronchiectasis: data from the European Bronchiectasis Registry (EMBARC)
Abstract
Introduction: Current bronchiectasis guidelines advise against the use of inhaled corticosteroids (ICS) except in patients with associated asthma, allergic bronchopulmonary aspergillosis (ABPA) and/or chronic obstructive pulmonary disease (COPD). This study aimed to describe the use of ICS in patients with bronchiectasis across Europe.
Methods: Patients with bronchiectasis were enrolled into the European Bronchiectasis Registry from 2015 to 2022. Patients were grouped into ICS users and non-users at baseline and clinical characteristics associated with ICS use were investigated. Patients were followed up for clinical outcomes of exacerbation, hospitalisation and mortality for up to 5 years. We evaluated if elevated blood eosinophil counts (above the laboratory upper limit of normal) modified the effect of ICS on exacerbations.
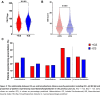
Results: 19 324 patients were included for analysis and 10 109 (52.3%) were recorded as being prescribed ICS at baseline. After exclusion of patients with a history of asthma, COPD and/or ABPA, 3174/9715 (32.7%) patients with bronchiectasis were prescribed ICS. Frequency of ICS use varied across countries, ranging from 17% to 85% of included patients. ICS users had more severe disease, with significantly worse lung function, higher Bronchiectasis Severity Index scores and more frequent exacerbations at baseline (p<0.0001). Overall, ICS users did not have a reduced risk of exacerbation or hospitalisation during follow-up, but a significant reduction in exacerbation frequency was observed in the subgroup of ICS users with elevated blood eosinophil counts (relative risk 0.70, 95% CI 0.59 to 0.84, p<0.001).
Conclusion: ICS use is common in bronchiectasis, including in those not currently recommended ICS according to bronchiectasis guidelines. ICS use may be associated with reduced exacerbation frequency in patients with elevated blood eosinophils.
Keywords: bronchiectasis.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: SA reports grants or contracts from any entity from Insmed, Chiesi, Fisher and Paykel and GlaxoSmithKline (GSK) ; royalties or licences from McGraw Hill; consulting fees from Insmed, Insmed Italy, Insmed Ireland, Zambon, AstraZeneca, CSL Behring, Grifols, Fondazione Internazionale Menarini, Moderna, Chiesi, MCD Italis, Brahms, Physioassist SAS, GSK; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from GSK, Thermofisher Scientific, Insmed Italy, Insmed Ireland, Zambon, Fondazione Internazionale Menarini; participation on a Data Safety Monitoring Board or Advisory Board from Insmed, Insmed Italy, AstraZeneca, MSD Italia. FCR reports grants or contracts from any entity from German Center for Lung Research (DZL), German Center for Infection Research (DZIF), IMI (EU/EFPIA) and iABC Consortium (including Alaxia, Basilea, Novartis and Polyphor), Mukoviszidose Institute, Novartis, Insmed Germany, Grifols, Bayer, InfectoPharm; consulting fees from Parion, Grifols, Zambon, Insmed and Helmholtz-Zentrum für Infektionsforschung; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from I!DE Werbeagentur, Interkongress, AstraZeneca, Insmed, Grifols, Universitätsklinikum Frankfurt am Main; payment for expert testimony from Social Court Cologne; support for attending meetings and/or travel from German Kartagener Syndrome and Primary Ciliary Dyskinesia Patient Advocacy Group Mukoviszidose; participation on a Data Safety Monitoring Board or Advisory Board—Insmed, Grifols and Shionogi; leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid—coordinator of the ERN-LUNG Bronchiectasis Core Network, chair of the German Bronchiectasis Registry PROGNOSIS, member of the SteerCo of the European Bronchiectasis Registry EMBARC, member of the SteerCo of the European Non-tuberculous Mycobacterial Pulmonary Disease Registry EMBARC-NTM, co-speaker of the Medical Advisory Board of the German Kartagener Syndrome and PCD Patient Advocacy Group, speaker of the Respiratory Infections and TB group of the German Respiratory Society, speaker of the Cystic Fibrosis group of German Respiratory Society (DGP), PI of the German Center for Lung Research, member of the Protocol Review Committee of the PCD-CTN, member of Physician Association of the German Cystic Fibrosis Patient Advocacy Group; other financial or non-financial interests—AstraZeneca, Boehringer Ingelheim, Celtaxsys, Corbus, Insmed, Novartis, Parion, University of Dundee, Vertex and Zambon. RD reports payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from LUPIN, CIPLA and Glenmark. CH reports payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from 30 Technology, CSL Behring, Chisi, Insmed, Janssen, LifeArc, Meiji, Mylan, Pneumagen, Shionogi, Vertex and Zambon. ML reports consulting fees from Armata, 30T, AstraZeneca, Parion, Insmed, Chiesi, Zambon, Electromed, Recode, AN2 and Boehringer Ingelheim; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Insmed; leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid on ERS Infection Group Chair. KD reports payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Novartis, Boehringer Ingelheim, GSK, Norma Hellas, Chiesi, AstraZeneca and Zambon; support for attending meetings and/or travel from Novartis, Boehringer Ingelheim, GSK, Norma Hellas, Chiesi, AstraZeneca and Menarini; participation on a Data Safety Monitoring Board or Advisory Board from Novartis, GSK and Chiesi. MLC reports consulting fees from Boxer Capital. ADS reports grants or contracts from any entity from AstraZeneca, Pfizer, GSK and Novartis; consulting fees from AstraZeneca, Insmed, GSK, Boehringer, 30T and Bayer; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from AstraZeneca, Pfizer, GSK and Novartis. MV reports grants or contracts from any entity from Chiesi; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Insmed and Publi Creation; support for attending meetings and/or travel from Zambon, Chiesi, Novartis, Behring and Gebro; participation on a Data Safety Monitoring Board or Advisory Board—Insmed; receipt of equipment, materials, drugs, medical writing, gifts or other services from Insmed and Novartis. PRB reports grants or contracts from any entity from GSK and Vertex; consulting fees from AstraZeneca, Chiesi, GSK, Insmed, MSD, Vertex, Viatris and Zambon; support for attending meetings and/or travel from AstraZeneca and Chiesi. SS reports honoraria for educational events, invited lectures and presentations supported by Sanofi, AstraZeneca, Medis, Berlin Chemie and Chiesi; participation on a Data Safety Monitoring Board or Advisory Board—AstraZeneca local advisory board. AB reports payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Chiesi; leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid—secretary of Assembly 5 (airway diseases, asthma, COPD and chronic cough), European Respiratory Society; vice-chair of Nordic Severe Asthma Network (NSAN)-NORDSTAR. Steering Committee of ERS CRC severe asthma, SHARP. PK reports support for attending meetings and/or travel from Nordic Respiratory Academy; participation on a Data Safety Monitoring Board or Advisory Board from Swedish Orphan Biovitrium; leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid from Finnish Respiratory Society and Finnish Tuberculosis Foundation Grant Committee; receipt of equipment, materials, drugs, medical writing, gifts or other services from Theravance. MM reports payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Pfizer; payment for expert testimony from Vertex; support for attending meetings and/or travel from Zambon; participation on a Data Safety Monitoring Board or Advisory Board—Zambon and Viatris. DO reports leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid—president of Serbian Society of Intensive Care Medicine. AA reports payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Zambon Group; support for attending meetings and/or travel from Zambon Group, Boehringer Ingelheim and Novartis Farma. EVB grants or contracts from any entity from Insmed, Boehringer Ingelheim and Zambon. MS reports consulting fees from GSK, Boehringer Ingelheim, Kamada and Zambon; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Insmed, Boehringer Ingelheim, GSK, AstraZeneca, Teva, Novartis, Kamada and Sanofi; support for attending meetings and/or travel from Novartis, Actelion, Boehringer Ingelheim, GSK and Rafa; participation on a Data Safety Monitoring Board or Advisory Board—Bonus Therapeutics, Israel; leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid—EMBARC Management, Israel Pulmonology Society Board, Israel Society for TB and mycobacterial diseases; receipt of equipment, materials, drugs, medical writing, gifts or other services from Trudell Medical; other financial or non-financial interests—associate editor, AM J Respir Crit Care Med. PG reports payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Insmed, GSK and Chiesi; support for attending meetings and/or travel from Chiesi; participation on a Data Safety Monitoring Board or Advisory Board—Boehringer, GSK and Pfizer. FB reports grants or contracts from any entity from AstraZeneca, Chiesi and Insmed; consulting fees from Menarini; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from AstraZeneca, Chiesi, GSK, Guidotti, Grifols, Insmed, Menarini, Novartis, OM Pharma, Pfizer, Sanofi, Viatris, Vertex and Zambon. EP reports grants or contracts from any entity from Grifols; consulting fees from Insmed, Bayer, Chiesi and Zambon; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Bayer, Chiesi, Grifols, GSK, Insmed, Menarini and Zambon; support for attending meetings and/or travel from Insmed, Pfizer and Moderna. JDC is supported by the GSK/Asthma and Lung UK Chair of Respiratory Research and reports grants or contracts from any entity from AstraZeneca, Boehringer Ingelheim, Genentech, Gilead Sciences, GSK, Grifols, Insmed, LifeArc and Novartis; consulting fees from AstraZeneca, Chiesi, GSK, Insmed, Grifols, Novartis, Boehringer Ingelheim, Pfizer, Janssen, Antabio and Zambon. All other authors report no conflicts of interest.
Figures