AMP-activated protein kinase mediates adaptation of glioblastoma cells to conditions of the tumor microenvironment
- PMID: 40122814
- PMCID: PMC11931870
- DOI: 10.1186/s13046-025-03346-2
AMP-activated protein kinase mediates adaptation of glioblastoma cells to conditions of the tumor microenvironment
Abstract
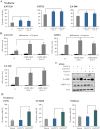
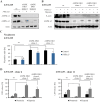
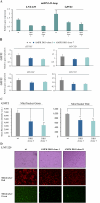
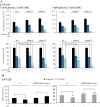
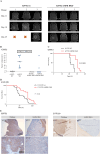
AMP-activated protein kinase (AMPK) is an energy sensor that regulates cellular metabolic activity. We hypothesized that in glioblastoma (GB), AMPK plays a pivotal role in balancing metabolism under conditions of the tumor microenvironment with fluctuating and often low nutrient and oxygen availability. Impairment of this network could thus interfere with tumor progression. AMPK activity was modulated genetically by CRISPR/Cas9-based double knockout (DKO) of the catalytic α1 and α2 subunits in human GB cells and effects were confirmed by pharmacological AMPK inhibition using BAY3827 and an inactive control compound in primary GB cell cultures. We found that metabolic adaptation of GB cells under energy stress conditions (hypoxia, glucose deprivation) was dependent on AMPK and accordingly that AMPK DKO cells were more vulnerable to glucose deprivation or inhibition of glycolysis and sensitized to hypoxia-induced cell death. This effect was rescued by reexpression of the AMPK α2 subunit. Similar results were observed using the selective pharmacological AMPK inhibitor BAY3827. Mitochondrial biogenesis was regulated AMPK-dependently with a reduced mitochondrial mass and mitochondrial membrane potential in AMPK DKO GB cells. In vivo, AMPK DKO GB cells showed impaired tumor growth and tumor formation in CAM assays as well as in an orthotopic glioma mouse model. Our study highlights the importance of AMPK for GB cell adaptation towards energy depletion and emphasizes the role of AMPK for tumor formation in vivo. Moreover, we identified mitochondria as central downstream effectors of AMPK signaling. The development of AMPK inhibitors could open opportunities for the treatment of hypoxic tumors.
Keywords: AMP-activated protein kinase; AMPK; Glioblastoma; Hypoxia; Metabolic adaptation.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: JPS has received honoraria for consulting or advisory board membership as well as travel or accommodation support from Abbvie, Medac, Novocure, Roche, Servier and UCB. MWR has received research funding from UCB as well as honoraria for advisory board participation from Alexion and Servier. All other authors declare that they have no competing interests.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

