Dopamine-driven increase in IL-1β in myeloid cells is mediated by differential dopamine receptor expression and exacerbated by HIV
- PMID: 40122818
- PMCID: PMC11931822
- DOI: 10.1186/s12974-025-03403-9
Dopamine-driven increase in IL-1β in myeloid cells is mediated by differential dopamine receptor expression and exacerbated by HIV
Abstract
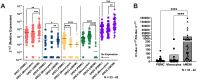
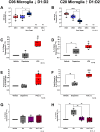
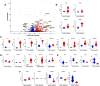
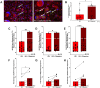
The catecholamine neurotransmitter dopamine is classically known for regulation of central nervous system (CNS) functions such as reward, movement, and cognition. Increasing evidence also indicates that dopamine regulates critical functions in peripheral organs and is an important immunoregulatory factor. We have previously shown that dopamine increases NF-κB activity, inflammasome activation, and the production of inflammatory cytokines such as IL-1β in human macrophages. As myeloid lineage cells are central to the initiation and resolution of acute inflammatory responses, dopamine-mediated dysregulation of these functions could both impair the innate immune response and exacerbate chronic inflammation. However, the exact pathways by which dopamine drives myeloid inflammation are not well defined, and studies in both rodent and human systems indicate that dopamine can impact the production of inflammatory mediators through both D1-like dopamine receptors (DRD1, DRD5) and D2-like dopamine receptors (DRD2, DRD3, and DRD4). Therefore, we hypothesized that dopamine-mediated production of IL-1β in myeloid cells is regulated by the ratio of different dopamine receptors that are activated. Our data in primary human monocyte-derived macrophages (hMDM) indicate that DRD1 expression is necessary for dopamine-mediated increases in IL-1β, and that changes in the expression of DRD2 and other dopamine receptors can alter the magnitude of the dopamine-mediated increase in IL-1β. Mature hMDM have a high D1-like to D2-like receptor ratio, which is different relative to monocytes and peripheral blood mononuclear cells (PBMCs). We further confirm in human microglia cell lines that a high ratio of D1-like to D2-like receptors promotes dopamine-induced increases in IL-1β gene and protein expression using pharmacological inhibition or overexpression of dopamine receptors. RNA-sequencing of dopamine-treated microglia shows that genes encoding functions in IL-1β signaling pathways, microglia activation, and neurotransmission increased with dopamine treatment. Finally, using HIV as an example of a chronic inflammatory disease that is substantively worsened by comorbid substance use disorders (SUDs) that impact dopaminergic signaling, we show increased effects of dopamine on inflammasome activation and IL-1β in the presence of HIV in both human macrophages and microglia. These data suggest that use of addictive substances and dopamine-modulating therapeutics could dysregulate the innate inflammatory response and exacerbate chronic neuroimmunological conditions like HIV. Thus, a detailed understanding of dopamine-mediated changes in inflammation, in particular pathways regulating IL-1β, will be critical to effectively tailor medication regimens.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures

Update of
-
Dopamine-driven Increase in IL-1β in Myeloid Cells is Mediated by Differential Dopamine Receptor Expression and Exacerbated by HIV.bioRxiv [Preprint]. 2024 Jun 10:2024.06.09.598137. doi: 10.1101/2024.06.09.598137. bioRxiv. 2024. Update in: J Neuroinflammation. 2025 Mar 23;22(1):91. doi: 10.1186/s12974-025-03403-9. PMID: 38915663 Free PMC article. Updated. Preprint.
Similar articles
-
Dopamine-driven Increase in IL-1β in Myeloid Cells is Mediated by Differential Dopamine Receptor Expression and Exacerbated by HIV.bioRxiv [Preprint]. 2024 Jun 10:2024.06.09.598137. doi: 10.1101/2024.06.09.598137. bioRxiv. 2024. Update in: J Neuroinflammation. 2025 Mar 23;22(1):91. doi: 10.1186/s12974-025-03403-9. PMID: 38915663 Free PMC article. Updated. Preprint.
-
Interleukin (IL)-34 promotes the inflammatory role of IL-1β-producing myeloid cells in pemphigus lesions.Br J Dermatol. 2025 Jul 17;193(2):287-297. doi: 10.1093/bjd/ljaf130. Br J Dermatol. 2025. PMID: 40203120
-
MRP8/14 Is a Molecular Signature Triggered by Dopamine in HIV Latent Myeloid Targets That Increases HIV Transcription and Distinguishes HIV+ Methamphetamine Users with Detectable CSF Viral Load and Brain Pathology.Viruses. 2023 Jun 13;15(6):1363. doi: 10.3390/v15061363. Viruses. 2023. PMID: 37376663 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Population-based interventions for reducing sexually transmitted infections, including HIV infection.Cochrane Database Syst Rev. 2004;(2):CD001220. doi: 10.1002/14651858.CD001220.pub2. Cochrane Database Syst Rev. 2004. Update in: Cochrane Database Syst Rev. 2011 Mar 16;(3):CD001220. doi: 10.1002/14651858.CD001220.pub3. PMID: 15106156 Updated.
References
-
- Abreu P, Llorente E, Hernández MM, González MC. Interleukin-1 beta stimulates tyrosine hydroxylase activity in the median eminence. NeuroReport. 1994;5(11):1356–8. - PubMed
-
- Ariffin JK, Sweet MJ. Differences in the repertoire, regulation and function of Toll-like Receptors and inflammasome-forming Nod-like Receptors between human and mouse. Curr Opin Microbiol. 2013;16(3):303–10. - PubMed
MeSH terms
Substances
Grants and funding
- T32-MH079785/T32-MH079785
- DA049227/DA/NIDA NIH HHS/United States
- DA057337/DA/NIDA NIH HHS/United States
- MH132466/MH/NIMH NIH HHS/United States
- R61 DA058501/DA/NIDA NIH HHS/United States
- R01 DA039005/DA/NIDA NIH HHS/United States
- R21 DA049227/DA/NIDA NIH HHS/United States
- K01 MH132466/MH/NIMH NIH HHS/United States
- R01 DA057337/DA/NIDA NIH HHS/United States
- T32 MH079785/MH/NIMH NIH HHS/United States
- DA058051/DA/NIDA NIH HHS/United States
- DA039005/DA/NIDA NIH HHS/United States
- A2003/WW Smith
LinkOut - more resources
Full Text Sources
Medical
Research Materials

