Extracellular PHF-tau modulates astrocyte mitochondrial dynamics and mediates neuronal connectivity
- PMID: 40122883
- PMCID: PMC11931834
- DOI: 10.1186/s40035-025-00474-9
Extracellular PHF-tau modulates astrocyte mitochondrial dynamics and mediates neuronal connectivity
Abstract
Background: Tau is an intracellular protein that plays a crucial role in stabilizing microtubules. However, it can aggregate into various forms under pathological conditions and be secreted into the brain parenchyma. While the consequences of tau aggregation within neurons have been extensively studied, the effects of extracellular paired helical filaments of tau (ePHF-tau) on neurons and astrocytes are still poorly understood.
Methods: This study examined the effect of human ePHF-tau (2N4R) on primary cultures of rat neuroglia, focusing on changes in neurites or synapses by microscopy and analysis of synaptosome and mitochondria proteomic profiles after treatment. In addition, we monitored the behavior of mitochondria in neurons and astrocytes separately over three days using high-speed imaging and high-throughput acquisition and analysis.
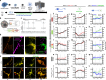
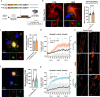
Results: ePHF-tau was efficiently cleared by astrocytes within two days in a 3D neuron-astrocyte co-culture model. Treatment with ePHF-tau led to a rapid increase in synaptic vesicle production and active zones, suggesting a potential excitotoxic response. Proteomic analyses of synaptosomal and mitochondrial fractions revealed distinct mitochondrial stress adaptations: astrocytes exhibited elevated mitochondrial biogenesis and turnover, whereas neuronal mitochondria displayed only minor oxidative modifications. In a mixed culture model, overexpression of tau 1N4R specifically in astrocytes triggered a marked increase in mitochondrial biogenesis, coinciding with enhanced synaptic vesicle formation in dendrites. Similarly, astrocyte-specific overexpression of PGC1alpha produced a comparable pattern of synaptic vesicle production, indicating that astrocytic mitochondrial adaptation to ePHF-tau may significantly influence synaptic function.
Conclusions: These findings suggest that the accumulation of PHF-tau within astrocytes drives changes in mitochondrial biogenesis, which may influence synaptic regulation. This astrocyte-mediated adaptation to tauopathy highlights the potential role of astrocytes in modulating synaptic dynamics in response to tau stress, opening avenues for therapeutic strategies aimed at astrocytic mechanisms in the context of neurodegenerative diseases.
Keywords: Astrocytes; Live imaging microscopy; Mitochondria; Synapse; Tau.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: For murine-derived primary cultures, experiments were performed according to an ethics protocol approved by our institutional review committee (CER-VD 2018–01622), Lausanne, Switzerland. Consent for publication: Not applicable. Competing interests: Not applicable.
Figures



References
-
- Wang Y, Mandelkow E. Tau in physiology and pathology. Nat Rev Neurosci. 2016;17:5–21. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

