Bemcentinib as monotherapy and in combination with low-dose cytarabine in acute myeloid leukemia patients unfit for intensive chemotherapy: a phase 1b/2a trial
- PMID: 40122885
- PMCID: PMC11930985
- DOI: 10.1038/s41467-025-58179-6
Bemcentinib as monotherapy and in combination with low-dose cytarabine in acute myeloid leukemia patients unfit for intensive chemotherapy: a phase 1b/2a trial
Abstract
Beyond first line, the prognosis of relapsed/refractory (R/R) acute myeloid leukemia (AML) patients is poor with limited treatment options. Bemcentinib is an orally bioavailable, potent, highly selective inhibitor of AXL, a receptor tyrosine kinase associated with poor prognosis, chemotherapy resistance and decreased antitumor immune response. We report bemcentinib monotherapy and bemcentinib+low-dose cytarabine combination therapy arms from the completed BerGenBio-funded open-label Phase 1/2b trial NCT02488408 ( www.clinicaltrials.gov ), in patients unsuitable for intensive chemotherapy. The primary objective in the monotherapy arm was identification of maximum tolerated dose with secondary objectives to identify dose-limiting toxicities, safety and efficacy, and bemcentinib pharmacokinetic profile. In the combination arm, the primary objective was safety and tolerability, with efficacy and pharmacokinetics as secondary objectives. Safety and tolerability were based on standard clinical laboratory safety tests and Common Terminology Criteria for Adverse Events version 4. Bemcentinib monotherapy (32 R/R, 2 treatment-naïve AML and 2 myelodysplasia patients) was well-tolerated and a loading/maintenance dose of 400/200 mg was selected for combination treatment, comprising 30 R/R and 6 treatment-naïve AML patients. The most common grade 3/4 treatment-related adverse events were cytopenia, febrile neutropenia and asymptomatic QTcF prolongation, with no grade 5 events reported. In conclusion, bemcentinib+low-dose cytarabine was safe and well tolerated.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: S.L. received travel support, advisory board honoraria and research funding from BerGenBio. She is currently supported by the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (Grant Agreement No. 758713) and by the Hector Stiftung II. M.H. is a consultant, advisor and/or speaker at AbbVie, Amgen, Bristol Myers Squibb, Certara, Glycostem, Jazz, Janssen, LabDelbert, Novartis, Pfizer, PinotBio, Servier and Sobi. His institution received research support from AbbVie, Agios, Astellas, BerGenBio, Bristol Myers Squibb, Glycostem, Jazz, Karyopharm, Loxo Oncology and PinotBio. D. Micklem, L.H.N., N. Madeleine, N. McCracken, C.O., C.G.-C. are employees at BerGenBio and may hold BerGenBio stock or stock options. The other authors declare no competing financial interests.
Figures
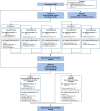
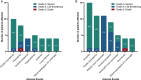

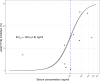
References
-
- Chien, L.-N. et al. Epidemiology and survival outcomes of acute myeloid leukemia patients in Taiwan: A national population-based analysis from 2001 to 2015. J. Formos. Med. Assoc.122, 505–513 (2023). - PubMed
-
- Döhner, H. et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood140, 1345–1377 (2022). - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

