Ulinastatin in the treatment of radiotherapy-induced oral mucositis in locoregionally advanced nasopharyngeal carcinoma: a phase 3 randomized clinical trial
- PMID: 40122906
- PMCID: PMC11930952
- DOI: 10.1038/s41467-025-57884-6
Ulinastatin in the treatment of radiotherapy-induced oral mucositis in locoregionally advanced nasopharyngeal carcinoma: a phase 3 randomized clinical trial
Abstract
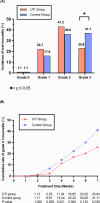
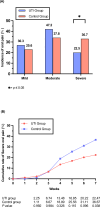
Radiotherapy-induced oral mucositis (RTOM) is a common side effect of radiotherapy in locoregionally advanced nasopharyngeal carcinoma (LA-NPC) receiving concurrent chemoradiotherapy (CCRT). In this phase 3 trial, we aim to evaluate the efficacy and safety of Ulinastatin (UTI) for the prevention and treatment of RTOM in LA-NPC patients (NCT03387774). The primary endpoint is the incidence of grade ≥3 acute RTOM during radiotherapy. Secondary endpoints include cumulative incidence of RTOM, recovery rate, the onset time and duration of grade ≥3 RTOM, oral pain (severe), safety and survival outcomes. 179 eligible patients are randomly assigned to UTI Group (n = 89) or Control group (n = 90). All UTI group patients complete UTI treatment as planned, and both groups complete scheduled CCRT. The incidence of grade 3 RTOM is significantly lower in UTI group compared with control group (25.8% vs 41.1%, P = 0.030). The trial meet its prespecified primary endpoint. No Ulinastatin related adverse events are observed during treatment. The 3-year overall survival (OS), locoregional relapse-free survival (LRRFS), distant metastasis-free survival (DMFS) and progression-free survival (PFS) in UTI group and control group are similar between two groups. In this work, Ulinastatin can effectively reduce the severity of RTOM and oral pain without increasing toxicity and compromising survivals.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests. Ethics approval: The study was approved by the Ethics Committee of Sun Yat-sen University Cancer Center (B2017-063-01-01) and registered on ClinicalTrials.gov, number NCT03387774.
Figures
References
-
- Chen, Y.-P. et al. Nasopharyngeal carcinoma. Lancet394, 64–80 (2019). - PubMed
-
- Al-Sarraf, M. et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: phase ||| randomized Intergroup study 0099. J. Clin. Oncol.16, 1310–1317 (1998). - PubMed
-
- Chen, L. et al. Concurrent chemoradiotherapy plus adjuvant chemotherapy versus concurrent chemoradiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma:a phase 3 multicentre randomised controlled trial. Lancet Oncol.13, 163–171 (2012). - PubMed
-
- Sun, Y. et al. Induction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a phase 3, multicentre, randomised controlled trial. Lancet Oncol.17, 1509–1520 (2016). - PubMed
-
- Zhang, Y. et al. Gemcitabine and cisplatin induction chemotherapy in nasopharyngeal carcinoma. N. Engl. J. Med.381, 1124–1135 (2019). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

