Long-Term Management and Therapeutic Sequencing for Patients with Relapsing Multiple Sclerosis in France: A Vignette Study
- PMID: 40122974
- PMCID: PMC12089545
- DOI: 10.1007/s40120-025-00726-w
Long-Term Management and Therapeutic Sequencing for Patients with Relapsing Multiple Sclerosis in France: A Vignette Study
Abstract
Introduction: We have analysed prescribing decisions for relapsing multiple sclerosis (RMS) of 111 neurologists ("participating physicians") in France using hypothetical case vignettes.
Methods: Six case vignettes were presented to participating physicians, each based on realistic, hypothetical clinical interactions between a neurologist and people with active or highly active RMS, with or without prior treatment with a disease-modifying therapy (DMT). "Disruptive events" are where the appearance of new MS disease activity, side-effects or other issues prompted the return of the hypothetical patients for a review of their care.
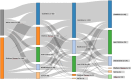
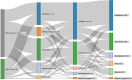
Results: A population of 111 participating physicians reviewed the cases and recommended treatments. Our data suggested a willingness among participating physicians to treat with higher-efficacy DMTs early in the course of RMS, with platform agents given to only one-quarter of DMT-naïve cases. MS disease activity was the main driver of switches to higher-efficacy DMTs, although an escalation approach was common in response to either moderate MS disease activity or side-effects on platform agents. A desire for pregnancy drove high usage of cladribine tablets and natalizumab (especially for cases negative for John Cunningham virus).
Conclusions: These findings suggest that the management of RMS in France has shifted in recent years towards a desire to achieve earlier and more effective control of disease activity for people with RMS. Better guidance on the sequencing of DMTs for different scenarios within the overall management of RMS may be warranted. This study offers valuable insights into the current practices of French neurologists in managing RMS, emphasizing the complexity of therapeutic decisions, the diversity of strategies, and the significance of an individualized approach in treatment management.
Keywords: Disease-modifying treatment; Relapsing multiple sclerosis; Tolerability.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: Patrick Vermersch received fees for consultancy, advisory board and scientific meetings from Biogen, Sanofi-Genzyme, Novartis, Teva, Merck Serono S.A.S, Lyon, France, an affiliate of Merck KGaA, Roche, Imcyse, AB Science, Janssen, Ad Scientiam and BMS, and research support. from Novartis, Sanofi-Genzyme, Roche and Merck. Xavier Moisset received financial support from Allergan-Abbvie, Aptis Pharma, Biogen, BMS-Celgène, Grünenthal, Lilly, Lundbeck, Teva, Merck Serono S.A.S, Lyon, France, an affiliate of Merck KGaA, Novartis, Orion, Pfizer, Roche, and Sanofi-Genzyme and non-financial support from SOS Oxygène unrelated to the current work. Jérôme de Sèze received fees for consultancy, advisory board and scientific meetings from Novartis, Biogen, Sanofi, Roche, Janssen, Merck Santé S.A.S, Lyon, France, an affiliate of Merck KGaA, Alexion, CSL Behring, Horizon Therapeutics, LFB, Sandoz, and Pfizer. Baptiste Roux and Anais Lecomte are employees of Société Fast4, Montpellier, France. Laura Luciani is an employee of Merck Santé S.A.S, Lyon, France, an affiliate of Merck KGaA. Martine Paret is an employee of Merck Serono S.A.S, Lyon, France, an affiliate of Merck KGaA. Ethical Approval: This study used fictional cases and does not fall within the scope of Articles L.1121–1 and following of the French Public Health Code. Given the nature of the study and the absence of real patient data, the protocol did not require submission to an Institutional Review Board (IRB) or Ethics Committees for approval. Therefore, IRB approval was not required. All participants were informed of the study objectives during recruitment phase. This communication was integral to ensuring that participants understood the purpose and significance of the study. Also, participants were made aware at this time that the results of the study would be published. Participants agreed to participate by providing verbal consent during the recruitment phase and were informed that they could withdraw from the study at any time without any consequences, ensuring their autonomy and comfort throughout the process. The independence of participants was verified as they completed the questionnaire autonomously on their personal devices, including phones, tablets, or computers, thus ensuring that their responses were not influenced by external factors. The protection of individuals’ data was maintained rigorously in accordance with the guidelines specified in the Information Security and Privacy Policy (ISSP). Detailed information regarding these protocols can be provided upon request.
Figures
References
-
- Montalban X, Gold R, Thompson AJ, et al. ECTRIMS/EAN Guideline on the pharmacological treatment of people with multiple sclerosis. Mult Scler. 2018;24:96–120. - PubMed
-
- Moisset X, Fouchard AA, Pereira B, et al. Untreated patients with multiple sclerosis: a study of french expert centers. Eur J Neurol. 2021;28:2026–36. - PubMed
LinkOut - more resources
Full Text Sources

