Light-Activated Pharmacological Tools for Exploring the Cholinergic System
- PMID: 40123150
- PMCID: PMC12131668
- DOI: 10.1002/med.22108
Light-Activated Pharmacological Tools for Exploring the Cholinergic System
Abstract
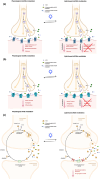
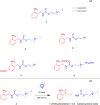
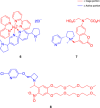
Cholinergic transmission plays a critical role in both the central and peripheral nervous systems, affecting processes such as learning, memory, and inflammation. Conventional cholinergic drugs generally suffer from poor selectivity and temporal precision, leading to undesired effects and limited therapeutic efficacy. Photopharmacology aims to overcome the limitations of traditional drugs using photocleavable or photoswitchable ligands and spatiotemporal patterns of illumination. Spanning from muscarinic and nicotinic modulators to cholinesterase inhibitors, this review explores the development and application of light-activated compounds as tools for unraveling the role of cholinergic signaling in both physiological and pathological contexts, while also paving the way for innovative phototherapeutic approaches.
Keywords: muscarinic acetylcholine receptors; nicotinic acetylcholine receptors; photopharmacology; photoswitch; uncaging.
© 2025 The Author(s). Medicinal Research Reviews published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
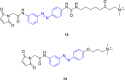
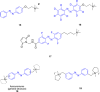
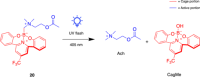
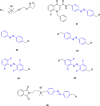
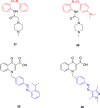
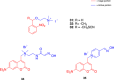
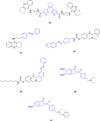
References
-
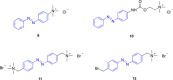
- Matera C., Quadri M., Sciaccaluga M., et al., “Modification of the Anabaseine Pyridine Nucleus Allows Achieving Binding and Functional Selectivity for the Α3β4 Nicotinic Acetylcholine Receptor Subtype,” European Journal of Medicinal Chemistry 108 (2016): 392–405, 10.1016/j.ejmech.2015.11.045. - DOI - PubMed

