An adamantane-based ligand as a novel chemical tool for thermosensory TRPM8 channel therapeutic modulation
- PMID: 40123199
- PMCID: PMC12220852
- DOI: 10.1111/febs.70065
An adamantane-based ligand as a novel chemical tool for thermosensory TRPM8 channel therapeutic modulation
Abstract
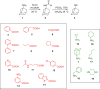
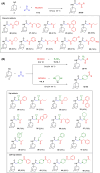
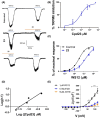
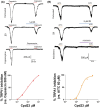
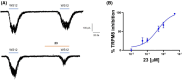
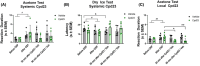
Transient receptor potential cation channel subfamily M member 8 (TRPM8) is a nonselective thermosensory cation channel expressed in peripheral nociceptor terminals where it transduces cold temperatures and cooling agents such as menthol. TRPM8 dysfunction has been involved in disabling sensory symptoms, such as cold allodynia. In addition, its widespread expression has signaled this channel as a pivotal therapeutic target for a variety of diseases, from peripheral neuropathies to cancer. Thus, the design and therapeutic validation of TRPM8 antagonists is an important endeavor in biomedicine. To address this, we used the multicomponent Passerini and Ugi reactions to design a novel family of TRPM8 modulators using as a scaffold the adamantane ring that exhibits drug-like qualities. These green chemistry transformations are ideal for the fast synthesis of libraries of medium complexity with minimal or no generation of waste by-products. We report the identification of a family of TRPM8 agonists and antagonists. Among them, 2-((3S,5S,7S)-adamantan-1-ylamino)-2-oxoethyl [1,1'-biphenyl]-2-carboxylate (referred to as compound 23) is a potent and selective antagonist that reduces TRPM8-induced neuronal firing in primary nociceptor cultures. Compound 23 exhibits 10-fold higher potency for human TRPM8 (hTRPM8) than for hTRPV1 and hTRPA1 channels. Notably, local administration of compound 23 significantly attenuated oxaliplatin-induced peripheral cold allodynia by modulating epidermal TRPM8 sensory endings. Thus, α-acyloxy carboxamide 23 appears as a promising therapeutic candidate to topically intervene on TRPM8-mediated peripheral neuropathies.
Keywords: cold allodynia; drug discovery; ion channel; medicinal chemistry; neuropathy.
© 2025 The Author(s). The FEBS Journal published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Kashio M & Tominaga M (2022) TRP channels in thermosensation. Curr Opin Neurobiol 75, 102591. - PubMed
-
- Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, Earley TJ, Dragoni I, Mcintyre P, Bevan S et al. (2002) A TRP channel that senses cold stimuli and menthol. Cell 108, 705–715. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

